One energetic oxidation reaction in alkenes occurs when there is a rupture of the molecule, that is, the simultaneous breaking of the two double bonds and the oxygen inlet in the organic molecule.
It is possible to carry out this type of reaction with alkenes when using potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7) concentrated, in an acidic medium, while hot.
In the acidic medium, there are H ions3O+ causing KMnO to decompose4 and releasing 5 atoms of nascent oxygen [O] for every 2 KMnO4. Note this decomposition below:

This reaction is called "energetic" because it breaks the two bonds of the double (in mild oxidation only the bond is broken pi), another factor is that this reduction of manganese in an acidic medium is much more intense than in a basic medium, as it is done in mild oxidation.
The nascent oxygen released then reacts with an alkene, but the final product depends on what the types of carbon that are doing the double bond, ie if they are primary, secondary or tertiary. See what happens in each case:
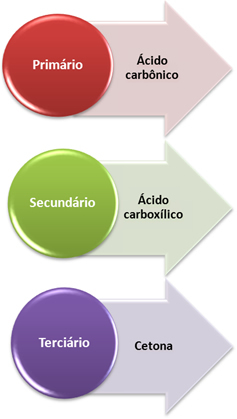
- Primary carbon: If the unsaturation is between two primary carbons, the products formed will be two carbonic acids (H2CO3). If only one of the carbons in the double bond is primary, only one of the molecules formed will be carbonic acid. However, this compound is unstable and has never been isolated, it breaks down into water and carbon dioxide.
- Secondary carbon: If the unsaturation is between two secondary carbons, the two products formed will be carboxylic acids. If only one of the carbons is secondary, it will give rise to a carboxylic acid molecule, while the other will depend on the other carbon.
- Tertiary carbon: Gives rise to ketone.
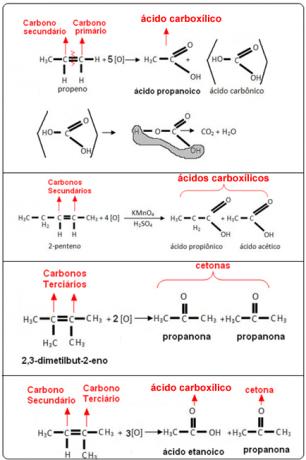
See the examples below:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidacao-energetica-alcenos.htm

