Transesterification reactions are those in which an ester is obtained through another ester. This method is quite viable because, since it takes place in only one step, it is processed quickly in the presence of a catalyst, it is simple, cheap and is carried out under ambient pressure.
The transesterification can be carried out in an acidic or basic medium and as it is also an equilibrium, alcohol is used as a solvent, which favors the formation of a new ester. This type of transesterification that reacts the ester with alcohol is called alcoholysis. But, you can also react the ester with a carboxylic acid or with another ester, being called acidolysis and interesterification, respectively.
Generically, the transesterification reaction can be represented by:
Currently, the most used basic and acid catalysts are sodium or potassium hydroxides and alkoxides, and sulfuric and hydrochloric acids.
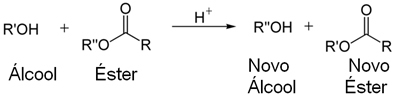
In the figure below, we have shown two examples of transesterification reactions, of the alcoholysis type, in which an ester reacts with an alcohol in an acidic medium and gives rise to another ester and another alcohol:

The main use of the transesterification reaction is to obtain the biodiesel. O biodiesel is a biofuel that can be used as fuel instead of diesel, but with the benefit of less polluting the environment, as it does not have sulfur element compounds, which are largely responsible for the aggravation of environmental problems, such as global warming, the greenhouse effect and rain acidic. Also, the biodiesel It is biodegradable, renewable and non-corrosive.
Do not stop now... There's more after the advertising ;)
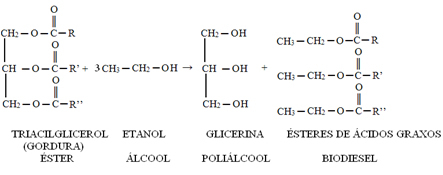
O biodiesel is a mixture of fatty acid methyl or ethyl esters. The transesterification reaction that gives rise to them consists of the reaction of triglycerides present in vegetable oils or animal fats with alcohol in the presence of a catalyst. The vegetable oils used can be castor oil, palm oil, soy palm, corn, peanuts, cotton, babassu etc. Frying oils can also be reused, this reuse is beneficial to the environment because it prevents these oils from being released into the waters of rivers, lakes, groundwater or contaminating the ground.

The alcohol used in this reaction has generally been methanol or ethanol. Thus, in addition to the biodiesel, you also get glycerin as a product. This is another positive aspect, because glycerin is a product with commercial value, being used in the production of cosmetics and cleaning products.
This transesterification reaction to obtain the biodiesel is represented below:
See the text Obtaining Biodiesel.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Transesterification Reactions"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/reacoes-transesterificacao.htm. Accessed on June 27, 2021.
Chemistry

Esters, Food Flavoring, Flavoring, Esterification Reaction, Methyl Anthranilate, Pentyl Acetate, Butyl Ethanoate, Ethyl Butanoate, Propanetriol, Glycerin, Stearin.