The synthesis of urea represented a milestone in the history of Organic Chemistry, as it was previously believed that organic compounds could only be produced by living beings, animals and plants. This idea was known as "Theory of Life Force" or “Theory of Vitalism”.
However, there was a German chemist and doctor called Friedrich Wohler (1800-1882), which in the year of 1828 carried out an experiment with the initial objective of preparing ammonium cyanate (NH4OCN(s)). He did this from two inorganic compounds, silver cyanide (AgCN(s)) and ammonium chloride (NH4Cl(s)).
First Wöhler heated silver cyanide in the presence of oxygen in the air, forming silver cyanate. This compound was then treated with an ammonium chloride solution, resulting in two products: a silver chloride precipitate and an ammonium cyanate solution.
After filtering and evaporating the ammonium cyanate solution, he obtained this substance in a solid state, which was heated, generating white crystals, that is, urea. The following are the chemical equations that represent the reactions that took place:
*Heating of silver cyanate in the presence of oxygen: AgCN(s) + ½ the2(g) → AgOCN(s)
* Treatment of silver cyanide with ammonium chloride: 2 AgOCN(s) + NH4Cl(here) → AgCl(ppt) + NH4OCN(here)
* Heating of solid ammonium cyanate: NH4OCN(s) → CO(NH2)2(s)
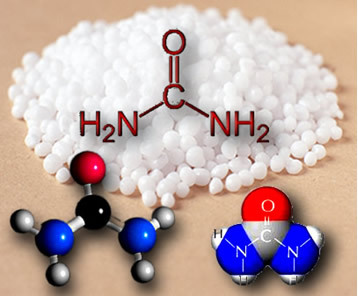
This reaction became known as Wohler's synthesis.
Do not stop now... There's more after the advertising ;)
Urea before was only obtained from urine, being an organic compound. Thus, the theory of vitalism fell to the ground, as Wöhler himself said in a letter he sent to his friend and research fellow Jöns Jacob Berzelius, who was the creator of this theory:
“I must inform you that I was able to prepare urea without the need for an animal's kidney, be it a man or a dog. The urea was obtained from an inanimate substance in a large glass balloon, which had nothing vital.” (WÖHLER apud USBERCO; SALVADOR, 2001, p. 15)
With this, the meaning of an organic compound changed: it was no longer one that originated from living organisms, but one composed of the element carbon with characteristic properties.
In addition, there was another fact that caught the attention of Wöhler and Berzelius: ammonium cyanate and urea had all the elements in the same amount, being two nitrogens, four hydrogens, one carbon and one oxygen. They then concluded that the difference between these two substances lay in the fact that the atoms of these elements were arranged in different ways in each.
Berzelius called these compounds isomers (from the greek iso means "equal" and mere means “parts”, that is, “equal parts”), which are compounds that have the same molecular formula and different structural formula.
USBERCO; J.; SAVIOR, E. Chemistry 3: Organic chemistry. Volume 3. P. 15.
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Urea synthesis"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/sintese-ureia.htm. Accessed on June 28, 2021.