In studying Newtonian Mechanics (Classical Mechanics), you may have noticed that knowing the starting position and the moment (mass and velocity) of all particles belonging to a system, we can calculate their interactions and predict how they will will behave. However, for Quantum mechanics, this process is a little more complex.
In the late 1920s, Heisenberg formulated the so-called uncertainty principle. According to this principle, we cannot precisely and simultaneously determine the position and momentum of a particle.
That is, in an experiment you cannot simultaneously determine the exact value of a particle's px moment component and also the exact value of the corresponding coordinate, x. Instead, the accuracy of our measurement is limited by the measurement process itself, in such a way that px. ∆x≥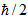 , where px is known as the uncertainty of ∆px, and the x position at the same instant is the uncertainty ∆x. On here
, where px is known as the uncertainty of ∆px, and the x position at the same instant is the uncertainty ∆x. On here  (It reads slashed h) is a simplified symbol for h/2n, Where H is Planck's constant.
(It reads slashed h) is a simplified symbol for h/2n, Where H is Planck's constant.
The reason for this uncertainty is not a problem with the apparatus used to measure physical quantities, but the very nature of matter and light.
So that we can measure the position of an electron, for example, we need to see it and, for that, we have to light it (basic principle of geometric optics). Furthermore, the measurement will be more accurate the shorter the wavelength of light used. In this case, quantum physics says that light is formed by particles (photons), which have energy proportional to the frequency of that light. Therefore, to measure the position of an electron we need to focus on it a very energetic photon, since the higher the frequency, the shorter the photon's wavelength.
However, to light the electron, the photon has to collide with it, and this process transfers energy to the electron, which will change its velocity, making it impossible to determine its momentum with precision.
This principle proposed by Heisenberg applies only to the subatomic world, since the photon energy transferred to a macroscopic body would not be able to change its position.
By Kléber Cavalcante
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/fisica/principio-incerteza.htm

