Rubber is a polymer that can be natural or artificial. Natural rubber is obtained through the latex, which is produced in many tropical plant species. But virtually all of the world's natural rubber production comes from latex extraction from Rubber tree(Hevea brasiliensis).
Incisions are made in the stem of this tree and the white liquid runs, being collected in bowls and must be collected frequently in order to avoid contamination and putrefaction.

The latex (natural rubber) is extracted from the rubber tree (Hevea brasiliensis)
Rubber polymer is an addition polymer, known as polyisoprene, as it is formed by the addition of 1,4 of isoprene monomers (methylbut-1,3-diene):
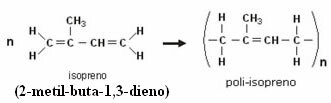
This reaction is here in a simplified way, because in trees they are much more complex and need enzymes acting as catalysts. The value of n in the polyisoprene formula above is on the order of 5000 and natural rubber is made up of about 35% of this polymer. See the polyisoprene macromolecule below:

However, natural rubber has some properties that make it difficult to use. For example, in cold weather it becomes hard and brittle, while in heat it becomes soft and sticky.
Therefore, it needs to go through a process called vulcanization, which was discovered in 1839 by Charles Goodyear. It is the addition of sulfur to polyisoprene, which breaks its double bonds and forms sulfur bridges that link the side chains and make hysteresis of the lower rubber (if it is squeezed, for example, it will quickly return to its original shape), low permanent deformation and large elasticity. In this way, rubber can be used to manufacture numerous products.
Mimicking the reaction that occurs in rubber trees, scientists started to carry out polymerization reactions with the addition of diene compounds, producing several types of synthetic rubbers. Depending on the type of monomer used to produce the polymer, rubbers with different properties are achieved.
The most common synthetic rubbers today are those obtained through the polymerization of acetylene (buta-1,3-diene), which forms the polybutadiene, and from chloroprene (2-chlorobut-1,3-diene), which produces the polychloroprene, or polyneoprene, or simply, neoprene:
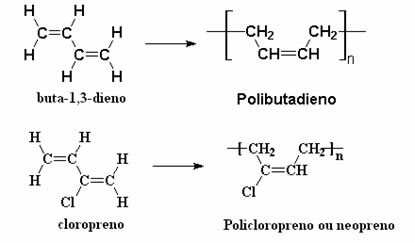
Currently, synthetic rubbers are used more than natural rubber. Both natural and synthetic are considered elastomers, that is, substances that, when subjected to tension, change from disordered rearrangements to linear arrangements, in a reversible manner.
The rubber used in tires is synthetic, known as Buna-S, being formed by erythrene (but-1,3-diene) and by styrene (vinylbenzene), which, in English, is written styrenehence the “S” at the end. The "na" also comes from the action of sodium (Na - from the Latin attrium) as a catalyst:
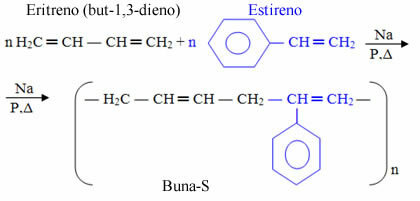
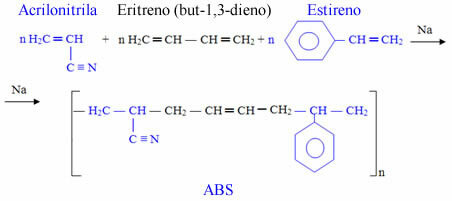
Buna-S is a copolymer, being formed by the addition of different monomers. Other examples of synthetic rubbers that are also copolymers is Buna-N, which is used in gas tank, hose and gasket linings, and the ABS, which is also used in the production of tires, telephones, electrical appliance casings and packaging.
Buna-N is formed by erythrene (but-1,3-diene), where the prefix “bu” comes from, and by acrylonitrile, where a nitrile group comes from and hence the “N” at the end. The "na" comes from sodium, which acts as a catalyst in the polymerization reaction of this copolymer:
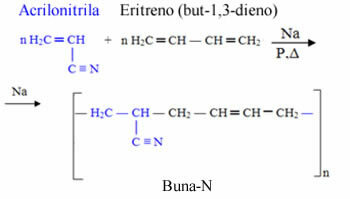
ABS is formed by the union of three monomers: acrylonitrile (A), but-1,3-diene (B) and styrene (S). styrene):
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/borracha-natural-sintetica.htm


