One of the biggest challenges for anyone learning Chemistry is called electronic distribution. What we are going to present in this context can greatly facilitate your studies related to the electron configuration proposed by scientist Linus Pauling.
The maximum number of electrons that fits in each layer or energy level is given through the table:
Energy Level Layer Maximum Number of Electrons
1st K 2
2nd L 8
3rd M 18
4th N 32
5° O 32
6th P 18
7th Q 8
The increasing order of energy of the sublevels is the order in the sequence of the diagonals. The Linus Pauling diagram is shown below:
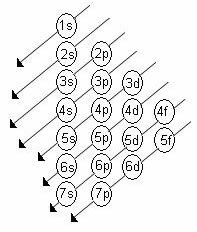
Basic rules:
1. The atomic number (Z) of the element, in the neutral state, indicates the number of electrons to be distributed. This number is represented in the lower left part of the element symbol.
2. Using the Pauling diagram, start distributing the electrons from top to bottom, taking into account the increasing order of energy (oriented by the direction of the arrows).
3. Fill the sublevels with the maximum level of electrons. If a given sublevel only holds 6 electrons, don't exceed that amount.
4. Check the number of electrons by adding them up in each sublevel.
Let's put it into practice?
Distribute the electrons of the iron atom (Z=26).
If the atomic number is 26, it means that in the normal iron atom there are 26 electrons. Applying the Pauling diagram, we will have:
Do not stop now... There's more after the advertising ;)

By Líria Alves
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Electronic Distribution Rules"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/regras-distribuicao-eletronica.htm. Accessed on June 27, 2021.