Using a Crookes ampoule, that is, closed glass tubes with a positive and a negative electrode, containing gases at extremely low pressures; the english scientist Joseph John Thomson (1856-1940) made an essential discovery for the evolution of the atomic model.
He subjected these gases to extremely high voltages, thus it was possible to observe the appearance of emissions, which were called cathode rays. Then, an external electric field was placed and, finally, it was verified that the cathode ray beam was deflected, always going in the direction and direction of the positively charged plate. Therefore, these emissions had negative charges.
Another important point is that no matter what gas was used, it was always the same; thus Thomson came to the logical conclusion that these negative charges were present in any and all matter, were an integral part of it. Thus, it was proved that, contrary to what Dalton had stated, the atom was not indivisible, as it had a negative subatomic particle, which was named
Sequentially, in 1886, another scientist, named Eugen Goldstein, discovered the anode rays or channels, which were positively charged rays, formed by what was left of the atoms of the gas that had their electrons ripped off by the electric discharge. These rays were known to possess positive charge because they were deflected in the opposite direction of the cathode rays, that is, they were attracted by the negative plate.
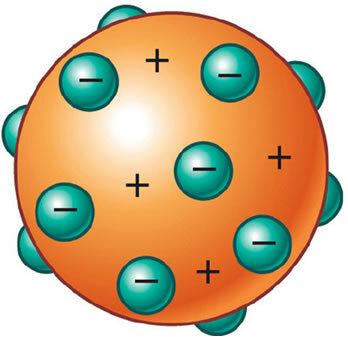
It was then discovered that the atom also had a positive part, which was even necessary to maintain its electrical neutrality. Thus, J. J. Thomson proposed a new model for the atom, dubbed the "raisin pudding" or "plum pudding". It would be a positively charged, non-massive sphere encrusted with (negative) electrons so that its total electrical charge is nil.
Do not stop now... There's more after the advertising ;)
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
General chemistry - Chemistry - Brazil School
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Thomson's Experiment with Electrical Discharges"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/o-experimento-thomson-com-descargas-eletricas.htm. Accessed on June 27, 2021.