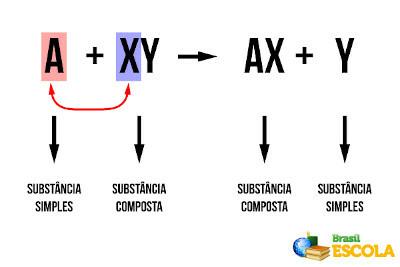
question 1
(PUC-MG) Under appropriate conditions, acetylene gas (C2H2(g)) and hydrochloric acid react to form vinyl chloride, C2H3Cl(g). This substance is used to produce polyvinyl chloride (P.V.C.) – plastic – and has recently been found to be carcinogenic. The reaction in the formation of C2H3Cl can be represented by the equation:
Ç2H2(g) + 1 HCl(here) → C2H3Cl(g)
When 2 mol of vinyl chloride are obtained, the volume of acetylene gas consumed in the CNTP (0°C and 1 atm) is equal to:
a) 11.2 L
b) 22.4 L
c) 33.6 L
d) 44.8 L
e) 89.2 L
question 2
The complete combustion reaction of acetone can be represented by the following equation:
Ç3H6O(v) + O2(g) → CO2(g) + H2O(v)
Consider this reaction carried out under a pressure of 100,000 Pa and at a temperature of 17 °C and calculate the volume of carbon dioxide, CO2(g), obtained at the complete burn of 3.0. 1026 propanone molecules, C3H6O(v).
a) 22.4 L.
b) 21.93 L.
c) 650.32 L.
d) 25 L.
e) 36180 L
More questionsEveryone already knows what fake news is. But how to deal with this theme in a newsroom like Enem? Discover the best strategies in this class.
In this video lesson, you will understand the transcription process of RNA, a very important nucleic acid for protein synthesis. Don't miss it!