When performing the nomenclature of organic compounds, one of the main difficulties encountered by Chemistry students is the nomenclature of the ramifications and the substituting organic radicals. In this text we will see which are the main groups of branches and radicals that appear in carbon chains and how to name them without errors.
Once the main chain has been chosen and numbered, all remaining chains are considered branches. The name of these branches must come before the name of the main chain, in alphabetical order and with the carbon number from which the branch is exiting.
However, the names of these branches must take into account two important pieces of information. Let's look at each of them:
1. The type of bond between the carbons:
In the case of saturated branches, that is, which have only single bonds between their carbons, the name will follow the following rule:

This means that their names are derived from the corresponding alkane, changing the ending ANO to IL or ILA (hence the generic name for alkyl or alkyl groups). See the example:
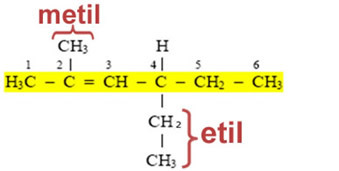
In addition to these organic groups that have only simple bonds between carbons; there are others that are derived from unsaturated compounds and aromatic compounds. The names of the principals are shown below:

2. Types of carbon in which the valence that connects the branch to the main chain is found:
In high school, it is also customary to study the branches that are linked to the main chain only through a simple link, which are called monovalent.
At this point it is interesting to know the difference between an organic radical and a substituent.
When an organic compound suffers a homolytic split, that is, a break in the bond between a carbon atom and a hydrogen atom in the chain, in which each atom takes one electron from the bond; we say that a radical. Thus, an organic radical is an isolated branch, which is not linked to the chain.
But radicals are highly unstable and reactive chemical species that can easily take the place of another atom (usually hydrogen) in the carbon chain. When that happens, it becomes a substitute.
usually use prefixes in the names of these substituents to better present their structure. The main ones are listed below:
- This: this prefix is used when the free valence is located on the primary carbon of a branched chain. It is typically used to identify radicals that have the following general structure:
H3Ç CH ─ (CH2)no─
|
CH3
Where n can take values of 0, 1, 2, 3, etc.
Examples:

- Sec- or s-: this prefix is used to indicate free valence on the secondary carbon. Examples:
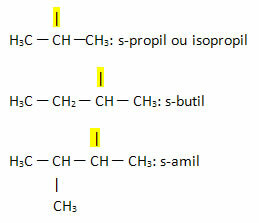
- Third- or t-: this prefix is used to indicate that the free valence is located on tertiary carbon.
Examples:

- Neo- or n-: this prefix indicates that the free valence is located on the primary carbon and generally indicates the presence of the following group:
CH3
|
H3Ç ─C ─ (CH2)no─
|
CH3
Examples:
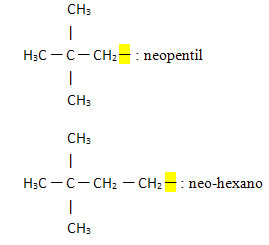
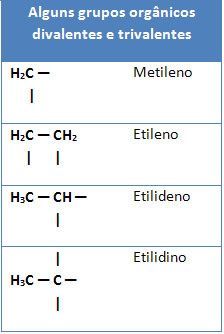
Below we also have some examples of organic groups divalent and trivalents:
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/nomenclatura-ramificacoes.htm


