Sulphonation reactions in alkanes they are organic substitution reactions, carried out with the aim of producing sulfonic acids (organic compounds that have the SO group3H attached to a carbon atom or to a carbon chain) and water (H2O).
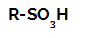
Structural formula of a sulfonic acid
For carrying out a sulfonation reaction on alkanes, we must mix an alkane (compound formed by a carbon chain saturated with only carbon and hydrogen atoms) and the sulfuric acid (H2ONLY4) concentrated, subjected to heating (∆).
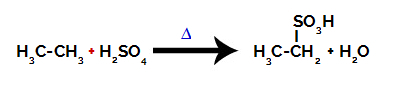
Chemical equation representing the sulfonation of a two-carbon alkane
Principles of the sulphonation reaction in alkanes
Such as alkane sulphonation reaction it is a substitution reaction, we have in it an exchange between smaller components electronegativity of the reactants, that is, between alkane and sulfuric acid. Alkane has hydrogen, and sulfuric acid, the sulfonic group.
Below, we have the step by step of the mechanism of a sulphonation reaction in alkanes. As an example, we will use the simplest alkane, methane (CH4):
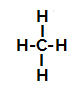
Structural formula of methane
1st Step: Disruption of the bond between hydroxyl (OH) and sulfur (S) in sulfuric acid.
Each hydroxyl group present in sulfuric acid is characterized by being more electronegative. With heating during the reaction, the tendency is that the bond between the hydroxyl group and the sulfur is broken:
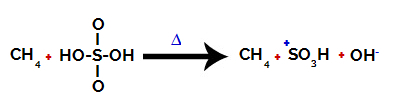
Disruption of the bond between sulfur and hydroxyl
However, the breaking of the bond of all hydroxyls does not occur because of the electronic rearrangement in the structure. A hydroxide anion (OH) is then obtained.-) and a sulfonic cation.
2nd Step: Hydroxyl group attack on the alkane molecule.
Then the hydroxy group (OH-) performs an attack on the alkane, causing the bond between carbon and hydrogen (which is more fragile because of the difference in electronegativity between them) to be broken.
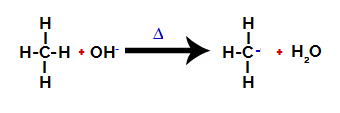
Breaking the bond between carbon and hydrogen in alkane
Note: Disruption of the bond between carbon and hydrogen will always occur most frequently on carbon of lower electronic density or charge. The fewer hydrogens carbon has, or the more groups attached to it, the lower its electron density. So we have:
Tertiary carbon < Secondary carbon < Primary carbon
After the bond is broken, the alkane becomes an electron deficient carbon (carbocation). The hydroxide group (OH)-) interacts with the released hydrogen and forms a water molecule.
3rd Step: Attack of the formed radical on the sulfonic group.
Finally, the sulfonic group is attacked by the rest of the alkane, forming a sulfonic acid.
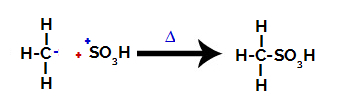
Structures that interact and form sulfonic acid
Examples of equations representing sulfonation reactions in alkanes
1st Example: Sulphonation of propane.
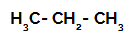
Structural formula of propane
Propane has two primary carbons and one secondary carbon, which have different charges because they are bonded to different amounts of hydrogen. Since carbon is more electronegative than hydrogen, these carbons have different electron densities.
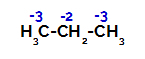
Distribution of charges on each carbon atom of propane
On primary carbons, the charge is -3 (because it is bonded to three hydrogens), and on the secondary carbon, the charge is -2 (because it is bonded to two hydrogens). Thus, there will be a break in the bond between carbon and hydrogen, sometimes at carbon 1 (of one molecule), sometimes at carbon 2 (of another molecule).
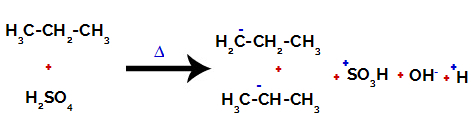
Breaking the bond between carbon and hydrogen on different carbons
After the breaks between the bonds, both in sulfuric acid and in alkane, there is the formation of products with the replacement of hydrogen on carbon 1 by a sulfonic group, and the same occurs on carbon 2.
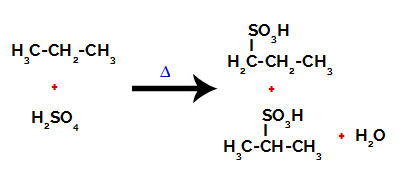
Products formed from the sulfonation of propane
2nd Example: Sulphonation of 2-methyl-butane.

Structural formula of 2-methyl-butane
2-Methyl-butane has three primary carbons, a secondary carbon and a tertiary carbon, which have different charges and, consequently, different electronic densities, as can be seen in the following structure:
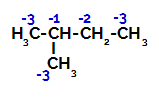
Distribution of electronic charges in 2-methyl-butane
Therefore, there are in 2-methyl-butane several possibilities of breaking the bond between carbon and hydrogen, which can occur on carbon 1 (of a molecule), carbon 2 (of another molecule), carbon 3 or carbon 4. However, it is noteworthy that the disruption at carbon number 2 is more common.
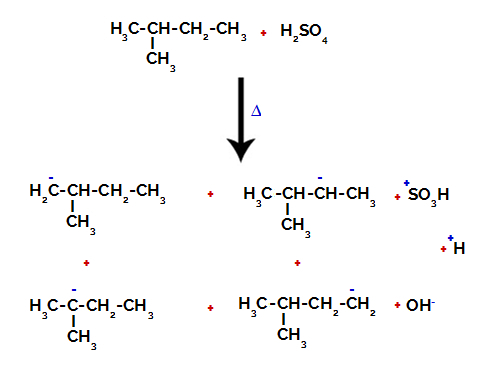
Breaking the bond between carbon and hydrogen on different carbons
After the breakage between the bonds, both in sulfuric acid and in alkane, the formation of products with the replacement of the hydrogen on carbon 1 by a sulfonic group, and the same happens on the carbon 2.

Products formed from the sulfonation of 2-methyl-butane
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-sulfonacao-alcanos.htm
