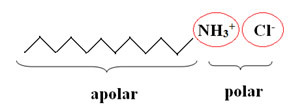
Hair conditioners, regardless of the brand, always have as their main compound a cationic surfactant.Surfactants or surfactants are compounds that have the ability to lower the surface tension of water. Furthermore, its molecules are characterized by a long non-polar chain and a polar functional group. The active part of the conditioners molecule is a cation (─NH3+):
Detergents (compounds that especially clean oil and grease dirt), including shampoo, are also surfactants, but of the anionic type. Thus, when a person uses the shampoo, their hair is electrostatically charged, due to the repulsion between the negatively charged molecules adhered to the hair. The negatively charged strands repel each other, tangling with each other and acquiring a rough, frizzy appearance.
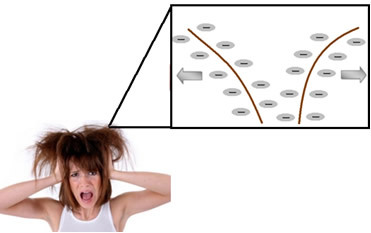
Therefore, it is necessary to apply the conditioner after washing your hair with shampoo. Generally, this compound has in its composition quaternary ammonium salt surfactants, as it has four groups linked to nitrogen with a positive charge. The most used is shown below:
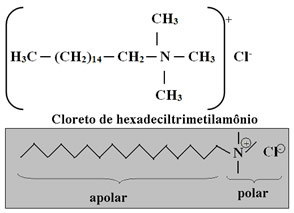
Due to its positive charges, the conditioner neutralizes the negative charges deposited on the hair by the shampoo, reducing the repulsion between the hairs. Positively charged ions adhere to the threads (and also fabrics), forming a uniform layer that has a strong attraction to water. That's why the strands are wetter, reducing the friction of the strands, making them easier to comb. Cationic surfactants also have a great affinity with the keratin of the hair strands, making them softer and more shiny.
Cationic surfactants have a bactericidal action. As they are irritating to the skin, they are not used in body care products, but only in hair creams and fabric softeners.
According to Brazilian legislation, fabric softeners and hair conditioners (cationic detergents) must have a minimum pH limit of 3.0 (acid).
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/condicionadores.htm
