As well as the cyclans, alkenes, alkynes, among others, the alkadienes also suffer the calls addition reactions. In the text about reactions in dienes, you will find that the addition reactions depend on the type of diene being worked on. The dienes can be broadly classified into:
condensed or accumulated (no single bond between the two doubles);
Ex: H2C = CH-CH3
conjugated or alternated (only a single bond between the two doubles);
Ex: H2C = CH—CH = CH2
isolated (at least two single bonds between the two doubles).
Ex: H2C = CH-CH2—CH2—CH = CH2
The Diels-Alder Reaction was developed in 1928 by two German chemists, Otto Paul Hermann Diels and Kurt Alder. This is a very important reaction within Organic Chemistry as it allows:
identify diene hydrocarbons;
obtain saturated cyclic hydrocarbons (preferably cyclohexanes).
This organic reaction only occurs in alkadienes or conjugated or alternated dienes and is called addition 1.4. The compound in which we can observe the 1,4 addition in a simpler way is but-1,3-diene, shown below:
H2C = CH—CH = CH2
NOTE: In this compound, we have the occurrence of resonance phenomenon, in which the electrons from the two pi bonds move through the chain. A pair of pi electrons starts to occupy the central region of the chain (between carbons 2 and 3), while the electrons from the other pi bond are shifted to one of the end carbons. Thus, we have binding sites on carbons 1 and 4 and a double between carbons 2 and 3.

Resonance in the but-2,3-diene structure
At Diels-Alder reaction, one of the reagents is a conjugated diene, while the other is an organic compound that can present a call double between carbon atoms. Below is an example of a Diels-Alder reaction between but-2,3-diene and propene:
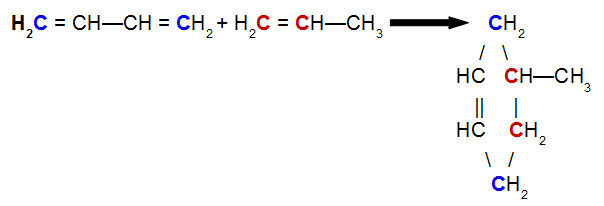
Equation of a Diels-Alder reaction of but-2,3-diene with propene
Analyzing the above equation, we can see that carbons 1 and 4 (both in blue) of but-2,3-diene bond respectively to carbons 1 and 2 (both red) of propene, giving rise to a branched cyclene, the 4-methyl-cyclohexene. This is due to the resonance in the but-2,3-diene molecule, which shifts a pi bond between carbons 2 and 3, and the breakage of the pi bond between carbons 1 and 2 of propene.
OBS.²: It is important to emphasize that, regardless of the compound that reacts with the conjugated alkadiene, there will be a break in the pi bond only between carbon atoms in the chain. If the structure has pi bonds between atoms other than carbon, they will not be broken. Below is an example of a structure that can react with a alkadiene in a Diels-Alder reaction, which results in the breaking of the pi bond between the carbons (in blue) of the double bond.
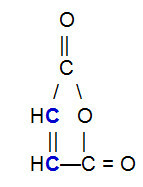
Structural formula of maleic anhydride
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-diels-alder.htm
