One oxidation reaction in organic compounds occurs when there is a oxygen inlet (or hydrogen output) in the organic molecule.
| One mild oxidation only occurs in hydrocarbons with unsaturation, that is, with double (alkenes) or triple (alkyne) bonds. |
For saturated hydrocarbons to oxidize, more energetic oxidation is needed.
Mild oxidation uses the Baeyer reactive, which corresponds to an aqueous solution of potassium permanganate (KMnO4) in a neutral or slightly alkaline (basic – OH1-), cold medium. This reactive is so called because German chemist Adolf Von Baeyer proposed a test, called the Baeyer's test, to identify alkenes and their cyclan isomers.
This test works as follows: as we will see later, an alkene reacts with the permanganate of potassium, thus its color, initially violet, becomes colorless and there is the appearance of a precipitate brown (MnO2). However, cyclans do not react with potassium permanganate. So if the solution remains violet, it's a cyclan.
The figure below shows that the test was positive for alkenes only in the left test tube, as the brown precipitate appeared.

The oxidation reaction of alkenes starts with the decomposition of the permanganate, producing oxygen:
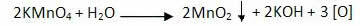
Mild oxidation of alkenes:
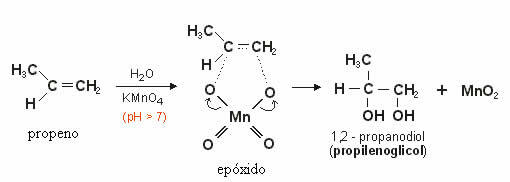
This produced oxygen will react with the double bond of the alkene forming a epoxide which later, through hydrolysis, becomes a alcohol or vicinal diol (glycol), that is, two OH groups on neighboring carbons.
In the example below we see the mild oxidation of propene:
Mild oxidation of alkynes:
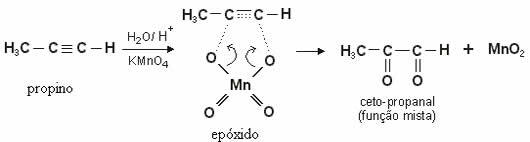
In the case of alkynes, the product formed will be diketones. With the exception of the ethyne (HC≡CH), where there are two hydrogens bonded on each carbon participating in the triple bond, a aldehyde.
Note the mild oxidation of propyne, with formation of a diketone:
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidacao-branda.htm
