In the addition reactionsin alkenes(hydrocarbons that have a double bond between two carbon atoms), a pi bond between two carbons is broken and each of the carbons attaches to a new atom. Look:
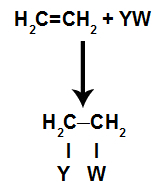
General schematic model of an addition reaction
Principles of an addition reaction
a) Breakage of pi link
A pi bond is easily broken because it is a weaker bond than a sigma bond. However, for this to happen, it is necessary that the alkene is subjected to conditions that provide this disruption. After breaking the pi bond between two carbons, a bonding site (for new atoms) always appears on each of the carbons involved.
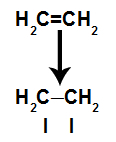
Schematic depicting pi link breakage
b) Conditions for breaking a pi bond
The factors that favor the breaking of a pi bond are:
Use of catalysts;
Heating;
Presence of an acid in the reaction medium.
c) The occurrence of the addition reaction
The binding sites created after the pi-bond is broken will always be occupied by atoms of the reactant that is in the same container as the alkene. The addition reaction is named after the type of reagent mixed with the alkene.
Types of Addition Reactions in Alkenes
a) Hydrogenation
An alkene is mixed with hydrogen gas (H2) in a container and subjected to the action of a solid catalyst (nickel, platinum or palladium) and heating (Δ).
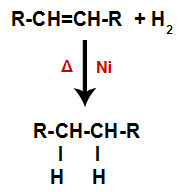
General scheme of a hydrogenation reaction in alkenes
The presence of the catalyst and heating cause the pi bond of alkene and the sigma bond between the hydrogens of H2 be broken more quickly. With that, we have the creation of two bonding sites in the alkene and two free hydrogen atoms in the reaction medium.

Creation of atom binding and separation sites
Thus, immediately afterwards, each free hydrogen atom occupies one of the bonding sites formed in the alkene. As the substance formed has only carbons and hydrogens, as well as only simple bonds between the carbons, it is a alkane.
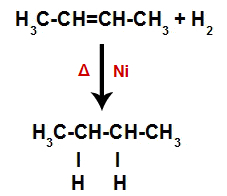
Equation representing the formation of alkane in hydrogenation
B) Halogenation
An alkene is mixed with simple substances (F2, Cl2, br2 Hey2) formed by halogens (chlorine, fluorine, iodine and bromine) in a container and subjected to the action of light (λ) and heating (Δ).

General scheme of a halogenation reaction in alkenes
The action of light and heating make the pi bond of the alkene and the sigma bond between substances formed by halogen to be broken more quickly. With this, we have the creation of two binding sites in the alkene and two free halogen atoms in the reaction medium.

Creation of atom binding and separation sites
Thus, immediately afterwards, each free halogen atom occupies one of the bonding sites formed in the alkene. As the substance formed has halogen linked to a structure composed of carbons and hydrogens, it is a organic halide.

Equation representing organic halide formation in halogenation
c) Hydration
An alkene is mixed with water (H2O) in a container and subjected to the action of a catalyst (in this case, sulfuric acid).
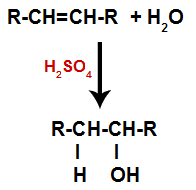
General scheme of a hydration reaction in alkenes
The presence of sulfuric acid in the reaction causes the pi bond of alkene and the sigma bond between the hydrogen (H) and the hydroxyl (OH) of water to break more quickly. With that, we have the creation of two bonding sites in the alkene and one free hydrogen and one hydroxyl in the reaction medium.
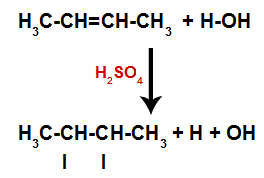
Creation of atom binding and separation sites
Thus, shortly thereafter, hydrogen and hydroxyl occupy one of the bonding sites formed in the alkene. As the substance formed has hydroxyl bonded to a saturated carbon (it only makes simple bonds), it is an alcohol.
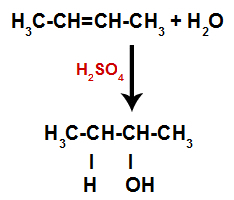
Equation representing the formation of alcohol in hydration
d) Addition with hydrogen halides
An alkene is mixed with a halogenated inorganic acid (HF, HI, HCl, HBr) in a container.

General scheme of an acid halide reaction in alkenes
The presence of acid in the reaction causes the pi bond of the alkene to break more quickly. The single bond in the acid is broken because these substances naturally ionize. Thus, two binding sites are created in the alkene and there is the presence of a free hydrogen and a halogen in the reaction medium.
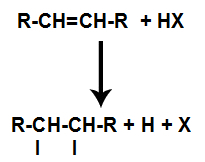
Creation of atom binding and separation sites
Thus, shortly thereafter, hydrogen and halogen occupy one of the bonding sites formed in the alkene. As the substance formed has halogen linked to a structure composed of carbons and hydrogens, it is an organic halide.
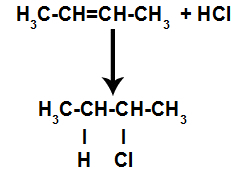
Equation representing organic halide formation in halogenation
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-adicao-alcenos.htm

