diffusion and effusion are two laws proposed by the Scottish chemist Thomas Graham, in the 19th century, based on his studies on gas mixtures and the behavior of gases when going through the walls of a container.
Among the observations made by Thomas Graham on the behavior of gases in relation to diffusion and effusion, we can highlight the following:
A gas always has a tendency to pass through small holes present in solid-state matter;
The bigger the molecule mass gas, the greater its difficulty in passing through the holes of solid matter;
The lower the gas density, the higher its movement speed;
The higher the temperature to which a gas is subjected, the greater the speed at which it spreads into the environment or passes through an orifice;
- A gas never remains static in a particular location.
Diffusion
Diffusion it is a physical phenomenon that consists in the ability of a gas to diffuse (that is, to spread) throughout the space of an area or inside a container. For this reason, we can say that, within a space, a gas is never confined to a single location.
Representation of the occurrence of a broadcast
In addition, we can define the diffusion still as being the capacity that one gas has to mix with another, when placed in the same container, forming a homogeneous gaseous mixture.
An example is when gases come out of vehicle exhausts. As toxic as they are, they do not cause short-term damage to humans, as they spread throughout the atmospheric air, consequently reducing their concentration.
Effusion
Effusion it is a physical phenomenon that consists in the passage of a gas through holes existing in the walls of a certain container, that is, it consists in the exit of a gas from one environment to another.
Representation of the occurrence of an outpouring
An example is when the balloons are inflated for a party and, the next day, they are all deflated, all because of the air escaping through the holes contained in the balloons.
Calculations related to diffusion and effusion
We can calculate, according to equations proposed by Graham, the speed at which a gas performs diffusion or effusion. According to Graham, the diffusion and effusion rates of two gases mixed in a container are always inversely proportional to the square of their relative densities or their molar masses.
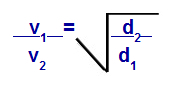
Graham's Law with regard to Density
v1 = velocity (effusion or diffusion) of gas 1 in the mixture;
v2 = velocity (effusion or diffusion) of the gas 2 in the mixture;
d1 = density of gas 1 of the mixture;
d2 = density of gas 2 of the mixture;
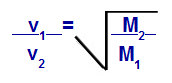
Graham's Law Concerning Molar Mass
v1 = velocity (effusion or diffusion) of gas 1 in the mixture;
v2 = velocity (effusion or diffusion) of the gas 2 in the mixture;
M1 = molar mass of gas 1 of the mixture;
M2 = molar mass of gas 2 in the mixture.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-difusao-efusao.htm

