Energy oxidation in alkynes is an organic reaction that occurs when an alkyne (a hydrocarbon that has a triple bond between two carbons) is added to an acidic solution with the reagent of baeyer (Potassium Permanganate - KMnO4).
NOTE: A reaction of energy oxidation can be performed with potassium dichromate (K2Cr2O7), and not just potassium permanganate.
Whenever a reaction from energetic oxidation in alkynes is carried out, the products that can be originated are carboxylic acids, water (the only item that appears in any of them) and carbon dioxide (CO2).
Baeyer Reagent in acidic medium
When Baeyer's reagent is mixed with water, in the presence of a acid (substance capable of releasing H ions+), there is the formation of two oxides (potassium oxide and manganese oxide II) and nascent oxygens ([O]).

Mechanisms of energy oxidation in alkynes
1st Mechanism: breaking the triple bond.
Initially, the triple bond is attacked by nascent oxygens formed by Baeyer's reagent. This attack causes the triple bond to be completely broken.
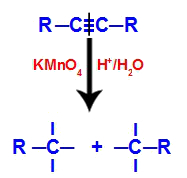
Disruption of the triple bond by the attack of nascent oxygens
When the triple bond is broken, the alkyne is split into two parts. On each of the carbons that were triple bonding, three free valences appear.
2nd Mechanism: Interaction of hydroxyl groups
Each of the free valences on the carbons where the triple bond was are occupied by hydroxyl groups (OH), forming a polyol (alcohol with several hydroxyls).
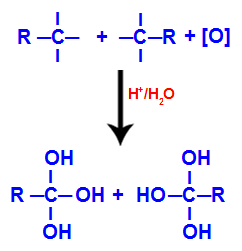
Formation of polyol with hydroxyl groups after triple bond cleavage
NOTE: If the carbon where the triple bond was has a hydrogen atom attached to it, that hydrogen atom will receive a nascent oxygen and also form another hydroxyl.
3rd Mechanism: Formation of water molecules
When a carbon has two or more OH groups, it becomes a very unstable structure due to the presence of extremely electronegatives. Thus, due to instability, the molecule undergoes self-dehydration, that is, a hydroxyl binds to hydronium (H+) from another hydroxyl and forms water.

Formation of water molecules from two hydroxyls present in the formed polyol
4th Mechanism: Product formation
After the formation of water molecules, the carbon loses a bond due to the exit of a hydroxyl, and the oxygen of the other hydroxyl also loses a bond that was being made with a hydrogen. Therefore, between this carbon and oxygen, a double bond appears, which forms a carbonyl (C=O) and stabilizes both.
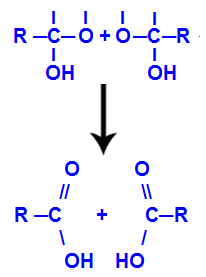
Carbonyl formation in the two structures obtained from alkyne
Example of energy oxidation equation in alkynes
Example: Energetic oxidation of But-1-ino
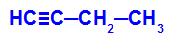
Structural formula of but-1-yne
When but-1-yne is placed in a medium containing Bayer's reagent, water and acid, the bond breaks. triple existing between carbons 1 and 2, due to the attack of the nascent oxygens existing in the middle, as in the equation bellow:
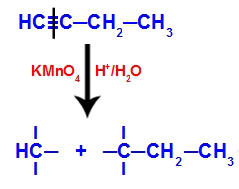
Breaking of the but-1-yne connections
After the triple bond is broken, carbons 1 and 2 receive three hydroxyls, but carbon 1 will have one more because it had a hydrogen atom (which binds to a nascent oxygen), forming two polyols (fragment 1 and 2).

Equation representing the formation of polyols
Soon after, as polyols are unstable, we have the formation of water molecules from the hydroxyls. In fragment 1, a water molecule appears (because there are three hydroxyls) and, in fragment 2, two molecules appear (because there are four hydroxyls).
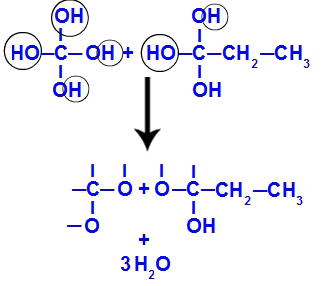
Formation of water molecules from but-1-yne fragments
Finally, we have the formation of the double bond between the carbons that have lost hydroxyl and the oxygens that have lost hydrogen, which gives rise to carbonyls.

Formation of carbonyls in the but-1-yne fragments
With the equation above, we can see that but-1-yne gave rise to a carboxylic acid and carbon dioxide (CO2).
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidacao-energetica-alcinos.htm