A electrosphere is the region of the atom in which the electrons They are located. The electrosphere, more precisely, is composed of atomic orbitals, determined by solving the Schrödinger equation. First determined by Rutherford's model, the electrosphere received significant advances during the validity of Bohr's atomic model.
The electrosphere can be divided into layers (or energy levels), as electrons have defined (non-continuous) energy. For atoms with two or more electrons, the shells divide into subshells (or subshells). The electrosphere is extremely important for understanding the properties of the atom and understanding the formation of chemical bonds.
Read too: How is the atom split?
Summary about the electrosphere
The electrosphere is the region of the atom in which electrons are located.
It is composed of atomic orbitals, wave functions that are solutions to Schrödinger's equations.
His concept began with the model of Ernest Rutherford.
Electrons are held in the electrosphere due to their attraction to the atomic nucleus.
The main advances in understanding the electrosphere occurred during the conception of Niels Bohr's model.
It is composed of layers (or energy levels), which are regions of defined energy.
For atoms with more than one electron, the shells divide into subshells (or subshells).
The electrosphere is important for understanding several properties, such as atomic similarity, stability, atomic radius, ionization energy, electron affinity, in addition to understanding the formation of bonds chemicals.
Video lesson on electrosphere
What is the electrosphere?
The electrosphere is defined as the region of the atomic structure in which electrons are located. In more in-depth interpretations, we say that it is composed of atomic orbitals, wave functions that are solutions to Schrödinger's equation. The mathematical expression of an atomic orbital, when squared, presents the probability density of the electron's location at a given point.
O concept of electrosphere began to emerge with the Ernest Rutherford's atomic model, which features electrons orbiting around a dense, positive nucleus. Later, Niels Bohr brought more significant interpretations of the electrosphere by mixing concepts from quantum mechanics.
Layers of the electrosphere
Electrons are held in the electrosphere due to their attraction to the atomic nucleus. However, it is known that these electrons are in shells whose energies are well defined. Such layers can also be called energy levels.
This conclusion came after spectroscopy experiments. For example, when an electric current is applied to gas H2 At low pressure, light is emitted by H2. In this condition, H ions are formed+ and electrons, which will return to the H ions+ and will form excited (energized) species of H+. To relieve excess energy, H ions+ release energy in the form of electromagnetic radiation (light) and recombine into H gas2 again.
You may remember that when white light passes through a prism, it breaks down into a continuous spectrum (similar to a rainbow); however, the same does not occur with the light coming from H2: when such radiation passes through the prism, only bright lines with a defined wavelength are observed in the H emission spectrum2, known as spectral lines.
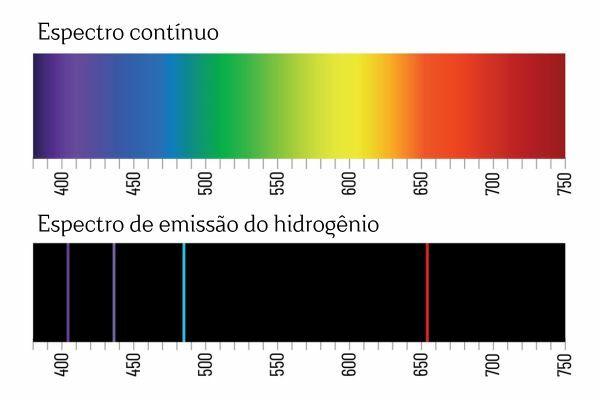
The interpretation for the emission spectra of the elements (with well-defined spectral lines) is that an electron, in an atom, cannot present any energy, but rather in well-defined quantities (so-called energy packets). If electrons did not have such energetic restrictions, the emission spectrum of the elements would be continuous, just like that of white light passing through a prism.
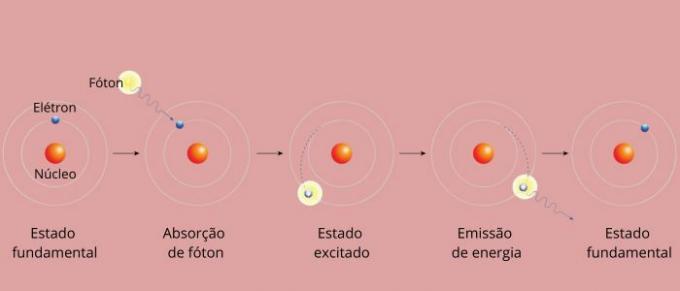
Each spectral line of an element became known as an energy level. (or layer, as we are more used to saying). These lines arise when an electron passes from one permitted energy level to another, in a process of energy change known as an electronic transition.
During the electronic transition, the electron passes from a lower energy level to a higher energy level. When returning to its initial level, it emits excess energy through electromagnetic radiation (light), giving origin to the spectral line whose energy value emitted is proportional to a value defined by the equation of Rydberg.
Johannes Rydberg was a Swedish spectroscopist who created an equation to define the trend of spectral lines based on the work of Swiss professor Johann Balmer. The specific energy of each layer is defined by solving the appropriate Schrödinger equation.
Each electronic layer has a number of electrons allowed. Currently, seven electronic layers are defined, identified by the letters K to Q, in alphabetical order, or by the letter n, where n ≥ 1. Thus, layer K is the layer where n = 1, and so on. The number of electrons allowed per shell is shown in the following table.
Energy level |
Layer |
Maximum number of electrons |
1 |
K |
2 |
2 |
L |
8 |
3 |
M |
18 |
4 |
N |
32 |
5 |
O |
32 |
6 |
P |
18 |
7 |
Q |
8 |
For hydrogenoid atoms (with only 1 electron, such as H, He+, Li2+), all atomic orbitals have the same energy (we call them degenerate orbitals); however, in atoms with two or more electrons, a very important effect arises, electron-electron repulsion. The consequence of this fact is that the orbitals of each layer begin to have different energies and, therefore, the layers begin to be described as sublayers (or sublevels).
For current atoms, each layer can be decomposed into up to four sublevels, represented by the letters “s” (from English, sharp), “p” (from English, main), “d” (from English, diffusal) and “f” (from English, fundamental).
Each sublevel supports a maximum number of electrons, defined by calculations and experiments. The “s” sublevel supports up to 2 electrons; the “p” sublevel, up to 6 electrons; the “d” sublevel, up to 10 electrons; and the “f” sublevel, up to 14 electrons. The K layer is the only one that only allows a single orbital and, therefore, only has a single sublevel.
Energy level |
Layer |
Sublevels |
1 |
K |
1s |
2 |
L |
2s, 2p |
3 |
M |
3s, 3p, 3d |
4 |
N |
4s, 4p, 4d, 4f |
5 |
O |
5s, 5p, 5d, 5f |
6 |
P |
6s, 6p, 6d |
7 |
Q |
7s, 7p |
Function of the electrosphere
The electrosphere of each atom can be used to explain various properties and behaviors of the atom.
Properties such as atomic radius, ionic radius, ionization energy and electron affinity have values that are a direct consequence of the electronic configuration of the electrosphere, more specifically the called valence shell, which is actually the outermost occupied electronic shell of an atom or ion.
A similarity between atoms from the same group in the Periodic Table is also a consequence of the electronic configuration of the valence shell. In chemical processes, we choose atoms from the same group on the Periodic Table as possible substituents, and this is only plausible, as these atoms have the same electronic configuration in the layer of valence.
To the chemical bonds, which occur between atoms to form ionic and covalent compounds (molecules), also occur through interactions between the electrospheres of atoms.
Read too: Schrödinger's atomic model — way of describing the atom using quantum mechanics
Relationship between the electrosphere and atomic structure
As noted, the electrosphere encompasses the region of the atom in which electrons can be found. Electrons, more specifically, are located in atomic orbitals, which have energy defined by quantum calculations.
The electrosphere is the largest region of the atomic structure, since the nucleus of an atom is very small. Thinking of the atom as a football stadium, the nucleus would correspond to a ball in the center of the field, while the rest of the stadium would be the electrosphere.
Nonetheless, in terms of mass, the electrosphere contributes little. As the mass of electrons is about 1836 times smaller than that of protons and neutrons, we can say that almost all of the atom's mass is concentrated in the nucleus.
Solved exercises on the electrosphere
Question 1
(Facisb 2023) In Bohr's model for the hydrogen atom, the electron can only occupy certain orbits. Some of these orbits are represented in the figure, where n refers to the energy levels that the electron has in each orbit.
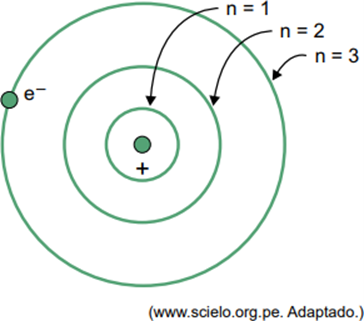
Consider that, in a hydrogen atom, the electron is in the orbit where n = 5.
According to the Bohr model, this electron will emit energy in the form of electromagnetic radiation only when
(A) make a transition to the orbit in which n is equal to 6.
(B) remain in the orbit where n = 5.
(C) transition to any orbit in which n is greater than 5.
(D) transition to any orbit in which n is less than 5.
(E) is ejected from the atom, ionizing it.
Answer: Letter D
When an electron is in an outer shell, upon returning to an inner shell with lower energy, it releases excess energy in the form of electromagnetic radiation (light). Therefore, the occurrence of light will only occur when the electron present in n = 5 makes a transition to an inner shell.
Question 2
(Uerj 2019) Recently, scientists managed to produce metallic hydrogen by compressing molecular hydrogen under high pressure. The metallic properties of this element are the same as the other elements in group 1 of the periodic classification table.
This similarity is related to the most energetic sublevel of these elements, which corresponds to:
(A) ns1
(B) n.p.2
(C) na3
(D) nf4
Answer: Letter A
The hydrogen atom has only a single electron, which is located in the first level, sublevel “s” (1s1). One reason why it is found in group 1 of the Periodic Table is because all other chemical elements in this group have atoms whose valence shell is of the same type (ns1). Therefore, due to a similar valence layer, hydrogen was able to be produced in this metallic form.
Sources:
DO CANTO, E. L.; LEITE, L. L. W.; CANTO, L. W. Chemistry – in everyday life. 1. ed. São Paulo: Moderna, 2021.
ATKINS, P.; JONES, L.; LAVERMAN, L. Principles of Chemistry: Questioning life and the environment. 7. ed. Porto Alegre: Bookman, 2018.
ATKINS, P.; DE PAULA, J.; KEELER, J. Atkins's Physical Chemistry. 11 ed. Oxford: Oxford University Press, 2018.
