White phosphorusis an allotropic form of the chemical element phosphorus, commonly represented by the molecular formula P4. Despite being called white phosphorus, its color is more reminiscent of wax or yellowish. White phosphorus is treated as the most stable form of phosphorus, although it is not the most thermodynamically stable.
White phosphorus has few direct uses in industry, being generally converted to the red allotrope, which has more direct uses and is less toxic. However, white phosphorus is used in military conflicts as a smoke-causing agent, for lighting and also to create fires, as white phosphorus ignites when in contact with air and at temperatures slightly higher than 30 °C.
Read too: What are the allotropic forms of sulfur?
Topics in this article
- 1 - Summary of white phosphorus
- 2 - What is white phosphorus?
- 3 - Composition of white phosphorus
- 4 - What is white phosphorus used for?
-
5 - Use of white phosphorus in war
- → White phosphorus bomb
- → Effects of exposure to white phosphorus
- 6 - Differences between white phosphorus and red phosphorus
White phosphorus summary
- White phosphorus is an allotropic form of phosphorus, represented by the formula P4.
- It has a yellowish color, close to the color of wax.
- It can be produced from phosphate rocks, sand and coke at high temperatures.
- It is very poorly soluble in water and ignites at temperatures just above 30°C.
- It is not widely used in industry, but it is the main elemental form of phosphorus produced.
- It is used in conflicts and wars as an agent that causes smoke, fires and for lighting.
- It has more direct uses than white phosphorus and is also less toxic than it.
What is white phosphorus?
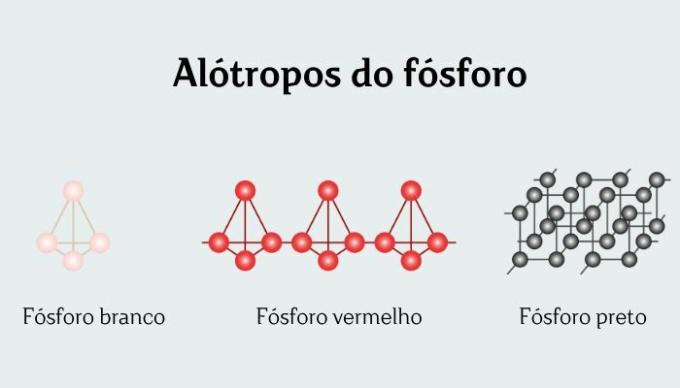
White phosphorus is one of the 12 allotropic forms that exist chemical element phosphorus, commonly represented as P4.
In ancient times, urine was the only known source for the element phosphorus and, therefore, all studies on this element made reference to production from urine. The phosphorus produced in this process had a white (or yellowish, wax-like) color, causing that white phosphorus was the first allotropic form of this element, also bringing it greater fame.
Posteriorly, In the second half of the 19th century, it was discovered that white phosphorus could be produced by heating phosphate rocks, with sand and coke. This method was improved for the modern way of producing white phosphorus, which consists of using a electric furnace (which reaches temperatures in the range of 1,400 to 1,500 °C) for heating a phosphate rock (Here3(DUST4)2), sand (silica, SiO2) and coke (carbon).
2Ca3(DUST4)2 + 6 SiO2 + 10 C → P4 + 6 CaSiO3 + 10 CO
Phosphorus vapors are condensed, forming a waxy solid that is insoluble in water.
Do not stop now... There's more after the advertising ;)
White phosphorus composition
White phosphorus is conventionally the most stable form of phosphorus, although it is, in fact, metastable (its heating leads to the thermodynamically more stable allotropic forms, red and black).
It appears in crystalline form as P molecules4 tetrahedral with P─P bonds of length equal to 221 pm. It is soluble in benzene, PCl3 and C.S.2, very little soluble in water. In fact, white phosphorus is stored in water to prevent it from oxidizing.
In humid air, it undergoes chemiluminescent oxidation, emitting a green glow and slowly transforming into P4O8 and the3.
What is white phosphorus good for?
White phosphorus has wide use in the military industry, as a smoke producer and for filling projectiles and grenades.
In industry in general, it is produced as elemental phosphorus from phosphate rocks, to then be converted into red phosphorus, another allotropic form, which has different chemical behavior. Red phosphorus has greater general application, being used to produce matchsticks, aluminum phosphide, flame retardants and for pyrotechnic purposes. White phosphorus is also the main way to produce higher purity compounds that may contain phosphorus. It also produces PCl3, through reaction with Cl2.
See too: After all, is the match in the box or on the stick?
Use of white phosphorus in war
White phosphorus ends up being used for military purposes, due to its self-ignition at low temperatures: above 50 °C (some authors say above 34 °C), in contact with air, white phosphorus ignites, producing phosphorus pentoxide (P4O10).
→ White phosphorus bomb
White phosphorus ignites at low temperatures. That's why, the use of white phosphorus as an incendiary weapon is common in conflicts. Its use is not based on lethality, but rather to create a fire, lighting or even a smoke screen to assist in the escape or retreat of troops. Smoke formation occurs when the temperature is sufficiently high in relation to the auto-ignition onset temperature of the allotropic form.

Because there are the aforementioned uses of white phosphorus (lighting, smoke screen and fire promotion), international conventions do not prohibit its use in conflicts. The UN Chemical Weapons Convention (CWC) does not consider it a chemical weapon. The only issue is that, if used to directly set people on fire at civilian targets, white phosphorus could violate Protocol III of the Convention on Certain Conventional Weapons (CCCW). However, this fact would need to be proven by an investigation by the competent bodies.
→ Effects of exposure to white phosphorus
Once ignited, white phosphorus fire is difficult to extinguish. Adheres very easily to skin and clothing, causing significant burns. Furthermore, even after initial treatment, white phosphorus can reignite if still in contact with air. Therefore, removing any trace of phosphorus from wounds is essential.
At first, washing with water and saline solutions is essential, as this can not only reduce the temperature, as well as stopping combustion and removing any trace of diluted phosphoric acid that may have been formed. The absorption of phosphorus by the burn can generate deviations in the body's calcium and phosphorus levels, which can quickly generate hypocalcemia and hyperphosphatemia, which may be the cause of fatal cardiac arrhythmia.
The smoke is extremely irritating to mucous membranes, where it combines with water to form phosphoric acid. Inhaling white phosphorus smoke causes irritation to the respiratory tract, generating cough, headache and delayed pulmonary edema.
White phosphorus is very toxic to humans and is quickly absorbed into the blood and liver. Ingestion of 100 mg can be fatal due to liver failure. Slow, prolonged exposure of small amounts also causes horrible consequences. The most common condition is match jaw, which affected several workers in phosphorus factories at the end of the 19th century and beginning of the 20th century.
Differences between white phosphorus and red phosphorus
White and red phosphorus are different allotropic forms of the chemical element phosphorus, both represented by P4.
Red phosphorus can be obtained from white phosphorus by heating it, in an inert atmosphere, at a temperature in the range of 540 K (267 °C). It was first obtained by Anton von Schötter in 1845.
There are several crystalline forms of red phosphorus. One of them is Hittorf (also known as violet phosphorus), which consists of a long and complex phosphorus chain.
Red phosphorus is less dense than white, has a higher melting point and is less reactive. Furthermore, red phosphorus is also not considered toxic, which makes it easier and safer to handle.
Sources
CHOU, T.-D.; LEE, T.-W.; CHEN, S.-L.; TUNG, Y.-M.; DAI, N.-T.; CHEN, S.-G.; LEE, C.-H.; CHEN, T.-M.; WANG, H.-J. The management of white phosphorus burn. Burns, n. 27, p. 492-497, 2001.
HOUSECROFT, C. AND.; SHARPE, A. G. Inorganic Chemistry. 2. ed. Pearson Education Limited: London, 2005.
Kelly, P. F. Phosphorus: Inorganic Chemistry. In: Encyclopedia of Inorganic Chemistry. 2. ed. Wiley: New Jersey, 2005.
WORLD HEALTH ORGANIZATION. White phosphorus. World Health Organization, 20 Oct. 2023. Available in: https://www.who.int/news-room/fact-sheets/detail/white-phosphorus.
Would you like to reference this text in a school or academic work? Look:
NOVAIS, Stéfano Araújo. "White phosphorus"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/fosforo-branco.htm. Accessed on November 9, 2023.