O atomic radius (r) is usually defined as half the distance between two nuclei of neighboring atoms, as the figure below represents:

atomic radius
The atomic radius differs from one atom to another according to its family and period on the Periodic Table. With respect to elements belonging to a same family, its atomic radius increases as the atomic number increases., that is, from top to bottom. For, in this sense, it means that from one atom to the other an energy level or electronic layer has increased, so its radius increases proportionally.
With regard to the element in the same period, that is, horizontally, the radius increases from right to left, or as the atomic number decreases. This is because they all have the same number of layers, what makes the difference is the amount of electrons in these layers, and the more electrons, the greater the attraction to the nucleus, thus decreasing the radius of the atom.

Atomic radius growth direction according to family and period in the Periodic Table
However, the atomic radius can vary depending on the connection that is made. Let's see how this happens:
*Ionic Bond: If the atom forms a cation, the atomic radius will decrease, since losing one or more electrons the nucleus will attract the electrons more intensely. Now if form an anion, ie gain electrons, the radius of the atom will increase, as the total charge of the electrosphere will be greater than the total charge of the nucleus, decreasing its attraction. The more electrons you gain or lose, the greater will be the variation in the size of the beam.
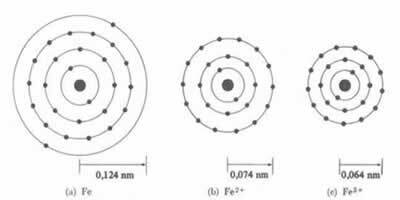
The diameter or radius of the cation is always smaller than the diameter or radius of the atom
Also, in a series of isoelectronic ions, which have the same amount of electrons and energy levels, the ion with the smallest atomic number will have a greater radius. For example, the ions 13Al3+, 12mg2+, 11At1+, 9F-1, 8O2- and 7N-3, all have 10 electrons and 2 electronic levels. But what has the largest radius is the 7N-3, because it has the smallest atomic number (Z= 7).
*Covalent bond: When two atoms make a covalent bond, if the two atoms are equal, as in the case of hydrogen gas (H2), one can speak of a covalent radius (r), which is half the length of the bond (d), that is, half the distance separating the two nuclei. Although, if the bond is made by different atoms, as in the case of hydrogen chloride (HCl), the length or distance (d) will be the sum of the covalent radii (r1 + r2) of the atoms involved in the covalence.
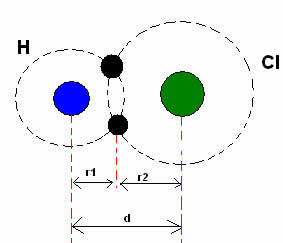
Sum of atomic radii in a covalent bond.
Of course, we must remember that this issue is much more complicated, as the covalent radius of an atom can vary as it bonds with other different atoms.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/variacao-raio-atomico-ligacoes-quimicas.htm

