A Zeroth Law of Thermodynamics is the law that collaborated in the conceptualization of greatness temperature and in the development of thermometers, based on his studies on the thermal balance between different bodies.
Read too: What is heat?
O What does the Zeroth Law of Thermodynamics say?
The Zeroth Law of Thermodynamics is the law that underlies the Thermodynamics for contributing to the definition of the physical quantity temperature, which is essential to understand the first and the second laws of thermodynamics. Because of this and because its development was later than the first two laws, it was named Law Zero by the physicist Ralph H. Fowler (1889-1944).
She can be stated as:
“If two bodies A and B are separately in thermal equilibrium with a third body, then A and B are in thermal equilibrium with each other.”
From this statement of the Zeroth Law, it is possible to understand that, if two bodies have the same temperature as a third body, then everyone will have the same temperature, then being in thermal equilibrium, in which there is no heat flow from one body to the other. other.
What is the Zeroth Law of Thermodynamics for?
The Zeroth Law of Thermodynamics is important because it defines the physical quantity temperature, which made it possible for thermometers to be manufactured. It can be observed in situations that refer to the thermal equilibrium between bodies, for example: when mixing water at different temperatures, there will be heat exchange until the waters reach the same temperature, and when entering a water with a higher or colder temperature, in a short time, the body will get used to the temperature due to the exchanges of heat.
Zeroth Law of Thermodynamics and Thermometers
The Zeroth Law of Thermodynamics contributed to the development of thermometers, which are devices used to measure the temperature of any body, living or not.

There are currently three types of thermometers that vary in their constitution and functioning:
analogues: compounds of Mercury;
Digital: formed by an electronic component on the tip that is temperature sensitive;
infrared digital: formed by infrared sensors, they measure the temperature without the need to touch the bodies.
Read too: What is the difference between temperature and heat?
What are thermometric scales?
To the thermometric scalesare representations of temperatures in different scales, the most used being the Celsius, Fahrenheit and Kelvin scales. We have below a comparison between the values of equivalent temperatures in these thermometric scales:
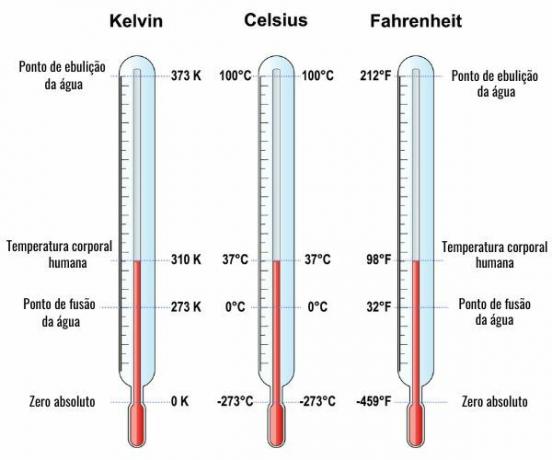
To find the temperature equivalence on different thermometric scales, the measured values of the temperatures in the boiling and melting points of water are marked and compared to a third point of which one wants to know the temperature. For that, he wasdeveloped the formula of equality between the different thermometric scales:
\(\frac{T_C}5=\frac{T_F-32}9=\frac{T_K-273}5\)
\(T_C\) is the temperature on the Celsius scale, measured in \([°C]\)
\(T_F\) is the temperature on the Fahrenheit scale, measured in \([°F ]\)
\(T_K\) is the temperature on the Kelvin scale, measured in \([K]\)
→ Video lesson on converting between thermometric scales
Solved exercises on the Zeroth Law of Thermodynamics
question 1
(Sailor's Apprentice) Three mercury thermometers are placed in the same liquid and, reaching thermal equilibrium, the graduate on the Celsius scale registers 45ºC. Thermometers graduated in the Kelvin and Fahrenheit scales, respectively, should record what values?
a) 218 K and 113 °F
b) 318 K and 113ºF
c) 318 K and 223 °F
d) 588 K and 313ºF
e) 628 K and 423 °F
Resolution:
Alternative B. First, let's convert the temperature in the Celsius scale to the temperature in the Kelvin scale using the formula that relates them:
\(\frac{T_C}5=\frac{T_K-273}5\)
\(TC=TK-273\)
\(45=TK-273\)
\(TK=273+45\)
\(TK=318\ K\)
Then, we will convert the temperature in the Celsius scale to the temperature in the Fahrenheit scale, using the formula that relates them:
\(\frac{T_C}5=\frac{T_F-32}9\)
\(\frac{45}5=\frac{T_F-32}9\)
\(9=\frac{T_F-32}9\)
\(9\cdot9=TF-32\)
\(81=TF-32\)
\(TF=81+32\)
\(TF=113\ ℉\)
question 2
(UERJ) Consider four objects A, B, C and D. It was observed that A and B are in thermal equilibrium with each other. Same for C and D. However, A and C are not in thermal equilibrium with each other. It can be concluded that:
a) B and D are at the same temperature.
b) B and D can be in thermal equilibrium, but they can also not be.
c) B and D cannot be at the same temperature.
d) The Zeroth Law of Thermodynamics does not apply in this case, because there are more than three objects.
e) A, B, C and D are at the same temperature.
Resolution:
Alternative C. Since bodies A and B are in thermal equilibrium, bodies C and D are also in equilibrium, but bodies A and C are not. in thermal equilibrium, then, according to the Zeroth Law of Thermodynamics, bodies B and D cannot be in equilibrium thermal.
By Pamella Raphaella Melo
Physics Teacher
Source: Brazil School - https://brasilescola.uol.com.br/fisica/lei-zero-da-termodinamica.htm
