At organic elimination reactions are those in which atoms or groups of atoms of a molecule are removed or eliminated from it, creating a new organic compound, in addition to an inorganic compound that is formed by the part that was deleted.
One type of elimination reaction is the dehydration, in which the molecule that is lost is water. The dehydration of alcohols (compounds that have the OH group attached to a saturated carbon in an open chain) can take place in two ways: intramolecular and intermolecular.
"Intra" means "inside", therefore, the intramolecular dehydration of alcohols occurs when there is an exit of a water molecule from “inside” the alcohol molecule itself. In this case, the organic product formed will be an alkene.
This reaction takes place only in the presence of a catalyst that acts as a desiccant, and most of the time it is concentrated sulfuric acid (H2ONLY4) and the temperature should be around 170ºC.
Example:
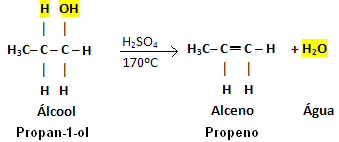
Note that the OH group left and hydrogen left the neighboring carbon, giving rise to water. Furthermore, the double bond that gave rise to the alkene was created.
The facility to suffer dehydration follows the following descending order:
Tertiary alcohols > Secondary alcohols > Primary alcohols
But what about when the OH group comes in the middle of the carbon chain? The hydrogen atom from which neighboring carbon atom will be released and form the water molecule?
For example, next is 2-methylpentan-3-ol. Note that one neighboring carbon atom is tertiary (highlighted in red), while the other is secondary (highlighted in blue):
H OH H
│ │ │
H3C─ Ç ─ Ç ─ Ç CH3
│ │ │
H H CH3
The hydrogen bonded to the tertiary carbon atom will be easier to leave, because its electronegative character is equal to δ+1Thus, the less negative the character of carbon, the weaker the bond between them and the easier it will be to break their bond.
In cases like this, all possible compounds are formed, however, the predominance will be given in the order: Tertiary alcohols > Secondary alcohols > Primary alcohols.
So we have:
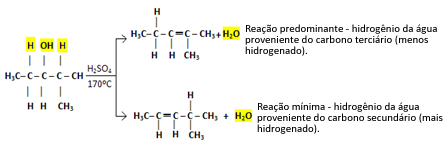
This type of reaction obeys the Saytzef's rule, which says that there will be a greater tendency for hydrogen to leave carbon less hydrogenated. This rule is exactly the opposite of Markovnikov's Rule used for addition reactions.
To complement your knowledge on this subject, also read the text "Intermolecular Dehydration of Alcohols”.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/desidratacao-intramolecular-dos-Alcoois.htm

