double salt is the name given to one of the classifications that the inorganic salts can receive. The other classifications are: simple, alum, hydroxylated, hydrogenated and hydrated. To be characterized as a double salt, an inorganic salt must have one of the combinations described below:
A cation (Y) and any two anions (X and W), and is represented by the following formula:
YXW
An anion (X) and any two cations (Y and Z), and is represented by the following formula:
YZX
Note: These salts are formed when a neutralization reaction is performed between two bases different and one acid, or between two different acids and a base.
Naming rule for a double salt
To name a double salt, it is necessary, first, to know its constitution, because, for each type of double salt, there is a specific naming rule, as can be seen below:
a) Nomenclature rule for double salt with two cations
When one double salt has two cations, we must use the following rule:
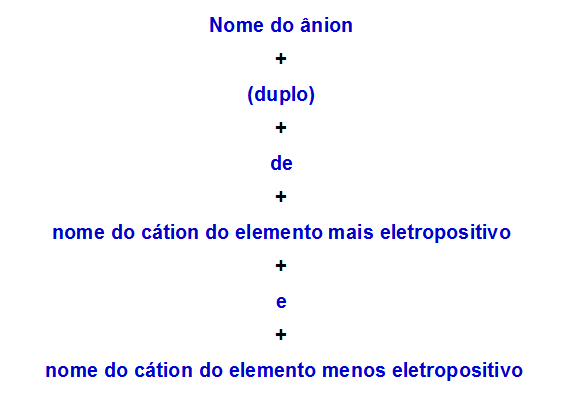
Nomenclature rule used for double salts with two cations
The following are two examples of application of this naming rule:
1st Example: Line4s
This salt is composed of:
Anion: sulfide (S-2);
More electropositive cation: lithium (Li+1);
Less electropositive cation: ammonium (NH4+1).
Hence, its name is lithium (double) ammonium sulfide.
2nd Example: RbCaBO3
This salt is composed of:
Anion: borate (BO3-3);
More electropositive cation: rubidium (Rb+1);
Less electropositive cation: calcium (Ca+2).
Thus, its name is borate (double) of rubidium and calcium.
b) Nomenclature rule for double salt with two anions
When one double salt has two anions, we must use the following rule:
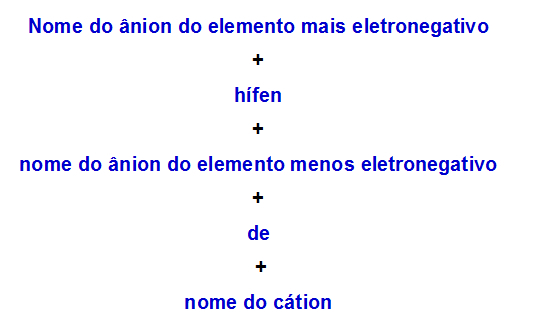
Nomenclature rule used for double salts with two anions
Here are two examples of applying this rule:
1st Example: MgFI
This salt is composed of:
Cation: magnesium (Mg+2);
Most electronegative anion: fluoride (F-1);
Less electronegative anion: iodide (I-1).
Therefore, its name is magnesium fluoride-iodide.
2nd Example: ZnNO2br
This salt is composed of:
Cation: zinc (Zn+2);
More electronegative anion: nitrite (NO2-1);
Less electronegative anion: bromide (Br-1).
Hence, its name is zinc nitrite-bromide.
Assembling the formula of a double salt from its nomenclature
a) For double salt with two cations
The construction of the formula of a double salt it depends on knowing its name, which is standardized as in all salt, that is, first the cation and then the anion. Since the double salt can have two cations, their order and placement in the formula follow the given name.
1st Example: barium nickel pyrophosphate II
This salt contains phosphate (P2O7), barium (Ba+2) and nickel II (Ni+2), cations written in that sequence. So its formula is BaNiP2O7.
2nd Example: copper II phosphate and gold I
This salt has phosphate (PO4-3), copper II (Cu+2) and gold I (Au+1), cations written in that sequence. Therefore, its formula is CuAuPO4.
b) For double salt with two anions
In case of double salt with two anions, we also follow, when placing the anions in the formula, the order as they appear in the given name.
1st Example: nickel sulfate-iodide III
This salt has sulfate anions (SO4-2) and iodide (I-1), written in this sequence, and the nickel III cation (Ni+3). So its formula is NiSO4I.
2nd Example: lead cyanide phosphate IV
This salt contains phosphate anions (PO4-3) and cyanide (CN-1), written in this sequence, and the lead cation IV (Pb+4). So its formula is PbPO4CN.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-um-sal-duplo.htm


