The buffer solution is usually a mixture of a weak acid and the salt of that acid, or a weak base and the salt of that base. This solution is intended to prevent very large variations in pH or pOH of a solution from occurring.
Below are some examples of buffer solutions:
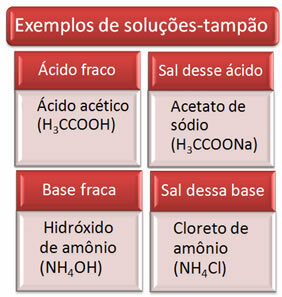
The effectiveness of the buffer solution can be seen in our blood, where, even with the addition of acid or base in small amounts to blood plasma, there is practically no change in its pH.
How does this happen, given that if we add acids or bases to water, its pH changes quickly?
Human blood is a slightly basic buffer system, that is, it is a buffered liquid: its pH remains constant between 7.35 and 7.45. One of the most interesting and important buffers in the blood is formed by carbonic acid (H2CO3) and by the salt of this acid, sodium bicarbonate (NaHCO3).
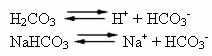
Thus, there are the following species in this buffer solution:
H2CO3: present in large quantities, as it is a weak acid, it suffers little ionization;
H+: from the ionization of H
HCO3-: also present in high quantities, from the ionization of H2CO3 and salt dissociation (NaHCO3);
At+: from the ionization of NaHCO3;
If a small concentration of acid is added to this solution, its ionization will occur, generating H cations+, which will react with HCO anions3- present in the medium, originating non-ionized carbonic acid. There is no change in pH.
If a base is added, OH anions will be generated-. These ions combine with the H cations+, from the ionization of H2CO3. Thus, the OH anions- are neutralized, maintaining the pH of the medium.
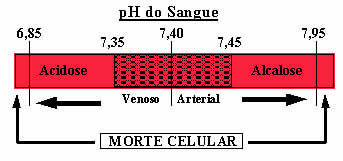
In addition to this buffer solution mentioned, there are also two other ones present in the blood, which are: H2DUST4/HPO42- and some proteins. If there were no such buffer solutions in the blood, the pH range could be seriously skewed. If the blood pH rises above 7.8, it is called alkalosis. If the pH drops too much, below 6.8, it will be a state of acidosis. Both are dangerous conditions that can lead to death.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/solucaotampao-no-sangue-humano.htm

