Among the complexities of atomic study is determining the size of the atom or, better, the atomic ray. This periodic property describes the distance from the nucleus to the outermost electron of its electronic levels. To determine it, an arithmetic average of the distance between the nuclei of two atoms that form a simple substance, for example, is performed.
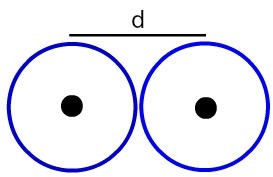
Representation of the distance between two atomic nuclei
Let's determine the atomic radius (RA) of the atoms exemplified in the image. To do this, just divide the distance between the cores by 2:
RA = d
2
O atomic ray study is important because it favors the understanding of some physical events (density, point of fusion, boiling point and ionization energy) and chemicals (chemical bonds) that occur with atoms.
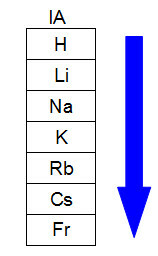
By analyzing a periodic table, we can assess whether an atom is larger or smaller in relation to another, and thus determine whether it has greater or lesser ease in having one or more electrons removed from their orbitals. THE evaluation and determination of the atomic radius in the periodic table
is performed according to two basic criteria:a) Number of energy levels (families or groups/vertical columns)
We know that atoms can have up to seven energy levels (K, L, M, N, O, P, Q) and that each chemical element is located in families or groups (vertical columns) and in periods (columns horizontal lines). Periods indicate the number of levels the element atom has, and the family indicates the most energetic sublevel of the atom. In a group or family, chemical elements differ by the amount of energy levels. See the table below:

The greater the number of energy levels of an atom, the greater its atomic radius. Analyzing the table above, it can be seen that francium has the largest atom because it has seven levels. The potassium atom, on the other hand, has a smaller radius as it has four energy levels. The following is a comparative representation between the francium atom and the potassium atom:
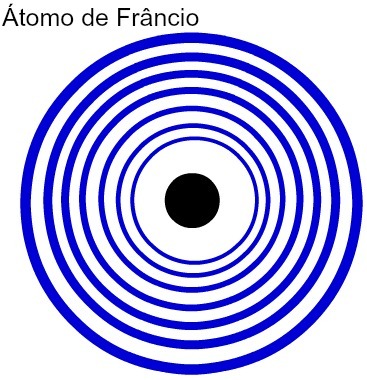
Representation of the seven energy levels of the Francium atom

Representation of the four energy levels of the Potassium atom
The following diagram represents how the increase of the atomic radius occurs in the same family or group (vertical columns) of the periodic table. The greater the number of levels, the greater the radius, that is, on the periodic table, the atomic radius grows from top to bottom:
Representation of how the atomic radius increases in a periodic table family
b) Atomic number (Z or number of protons) in the same period (horizontal column)
When chemical elements belong to the same period, their atoms have the same amount of energy levels, but the amount of protons inside their nuclei is different. The following is a sequence of elements belonging to the fourth period of the periodic table:
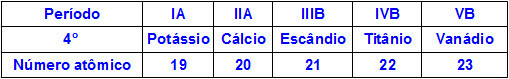
All atoms of the elements represented in the table above have four energy levels, but each of them has a different amount of protons in their nuclei. As the protons inside the nucleus exert an attractive force on the electrons present in the energy levels, the greater the amount of protons in the nucleus, the greater their attraction towards electrons. The result is an approximation of the levels towards the nucleus, decreasing the size of the atom.
↑Z = ↓Atomic radius
↓Z = ↑Atomic radius
Thus, we can state that the atomic radius of the element potassium is greater than that of vanadium due to the smaller number of protons.
The following diagram represents how the increase in atomic radius occurs in the same period (horizontal line). The smaller the atomic number, the larger the radius, that is, the atomic radius in the table grows from right to left
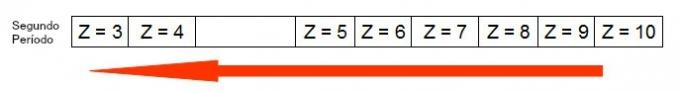
Representation of how the atomic radius increases in a period of the periodic table
By Me. Diogo Lopes Dia
