At ordinary batteries are called Leclanché batteries named after its inventor, the French chemist George Leclanché (1839-1882).

George Leclanché (1839-1882)
Leclanché created this type of battery in the year 1866. She is also called dry cell because until then there were only batteries that used aqueous solutions, such as Daniell's pile.
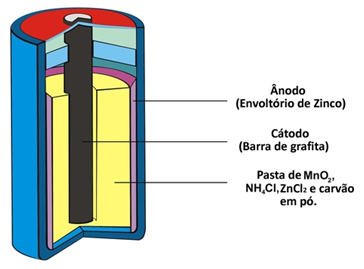
Two other names that this type of pile receives due to its constitution is acid pile or zinc carbon battery. It is basically formed by a zinc envelope separated from the other chemical species that make up the pile by means of a porous paper.
This zinc corresponds to the negative pole of the cell or anode, as it oxidizes, losing two electrons, according to the semi-reaction below:
Negative Pole - Anode: Zn (s) → Zn2+(here) + 2 and-
This pile also has a positive pole, the cathode, which is a graphite bar installed in the middle of the pile surrounded by manganese dioxide (MnO2), powdered charcoal (C) and a wet paste containing ammonium chloride (NH4Cl), zinc chloride (ZnCl2) and water (H2O).
Ammonium chloride and zinc chloride are salts with an acidic character, hence the name “acid cell”.
The graphite bar conducts the electrons lost by zinc to manganese, reducing manganese dioxide (MnO)2) to manganese trioxide (Mn2O3), according to the following semi-reaction:
Positive pole - Cathode: 2 MnO2(aq) + 2 NH41+(here) + 2e- → 1 Mn2O3(s) + 2NH3(g) + 1 hour2O(1)
The wet paste acts as a salt point, allowing the migration of hydroxyl anions (OH-) from graphite to zinc.
The overall reaction is given by:
Zn (s) + 2 MnO2(aq) + 2 NH41+(here) → Zn2+(here) + 1 Mn2O3(s) + 2NH3(g)
As such, these batteries are not rechargeable as all the manganese dioxide is converted to manganese trioxide. When the battery stops working, it must be discarded.
The ddp of these batteries is 1.5V. However, ammonia (NH3(g)) formed at the cathode can be deposited on the graphite bar, hindering the passage of electrons and reducing the voltage of the battery. To return to normal operation, just leave the battery to rest outside the device, as the zinc cation (Zn2+(here)) formed at the anode reacts with ammonia, leaving the graphite bar free.
In addition, placing the pile in the refrigerator can also help, as lowering the temperature favors the solubility of ammonia in the moist paste in the pile.
Another important factor about Leclanché dry cells that we must consider is that the zinc casing can corrode and thus leak the corrosive material and damage the device.. Therefore, the best thing to do is not to leave the batteries inside devices that are not used continuously.
Dry batteries are indicated for equipment that requires light and continuous discharge, such as remote control, wall clock, portable radio and toys.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/pilha-seca-leclanche.htm

