At substances are those materials with constant chemical composition and that have their physical properties well defined, such as melting and boiling points and the density, not varying in certain temperature and pressure.
The distilled water used in laboratories, for example, is a pure substance formed only by H molecules2O (this is its constant chemical composition) and, under a pressure of 1 atm (at sea level), it will always have a melting point equal to 0°C, a boiling point equal to 100°C and a density of 1.0 g/mL at 4°C. See two more examples:
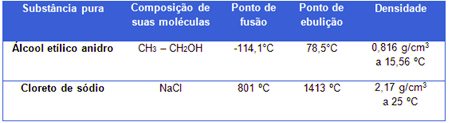
Physical properties of alcohol and sodium chloride
There are two types of substances, simple and compound:
The) simple substances
Are those whose molecules are formed only by a single type of chemical element. The atoms of the elements can appear in isolated form, being monoatomic substances, or form diatomic and triatomic molecules. Examples:
Monoatomic: is the case of helium gas (He), a prime gas that appears isolated in nature, and also from the
iron (Fe) and of the aluminum (Al), which are metals. See the text Metallic Connection to understand how the atoms of these elements stay together;Diatomics: the oxygen gas present in atmospheric air is made up of molecules each formed by two oxygen atoms, O2, and hydrogen gas molecules are formed by two hydrogen atoms, H2;
Triatomics: O ozone gas is formed by three oxygen atoms, O3.
B) compound substances
Are those whose molecules, or ionic clusters, are formed by two or more chemical elements or ions. Water, alcohol and sodium chloride, mentioned above, are all classified as composite substances or chemical compounds, as they are made up of different elements (hydrogen, oxygen, carbon, sodium and chlorine).
Another examples: carbon dioxide (CO2), carbon monoxide (CO), methane (CH4) and ammonia (NH3).
Compound substances can be broken down into simple substances. For example, when we pass an electric current over molten sodium chloride, a redox reaction which will give rise to two simple substances, metallic sodium (Na(s)) and chlorine gas (Cl2(g)). This process is known as Igneous Electrolysis of Sodium Chloride.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/substancias-simples-compostas.htm
