THE mild oxidation in alkenes is an organic reaction performed when a given alkene is placed in a medium formed by a base with water and potassium permanganate salt (KMnO4), salt that is commonly called Bayer reagent.
The product formed is called alcohol vicinal, that is, alcohols that have two hydroxyl groups (OH-) positioned on neighboring carbons, as in the following general formula:
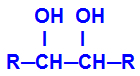
NOTE: R can be a radical or hydrogen atoms.
Bayer Reagent
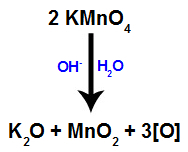
When the Bayer's reagent (KMnO4) is mixed with water in the presence of inorganic base (OH-), it reacts and forms potassium oxide (K2O), manganese dioxide (MnO2) and nascent oxygens (free oxygen, represented by [O]). See the balanced equation of the process.

These nascent oxygens formed from Bayer's reagent are responsible for the mild oxidation reaction in alkenes (as we will see in the following items).
Mechanisms in a mild oxidation reaction in alkenes
1st mechanism: formation of nascent oxygens from Bayer's reagent;
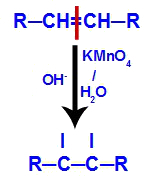
2nd mechanism: Attack of nascent oxygens to the double bond present in the alkene, causing the disruption of the pi bond and consequent formation of a free valence in each of the carbons involved with the pi link.
3rd mechanism: Nascent oxygens unite with hydroniums (H+) formed from the self-ionization of water, giving rise to hydroxyl groups (OH-).
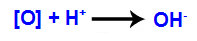
4th mechanism: Connection of the hydroxyls formed in each of the free valences located on the carbons where the pi bond was, originating a vicinal dialcohol.

Examples of mild oxidation reactions in alkenes
→ Mild oxidation reaction on but-2-ene
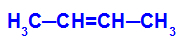
Structural formula of but-2-ene
Initially, the double bond between carbons 2 and 3 of the alkene is broken by the attack of nascent oxygens, creating a free valence (vertical trace) on carbons 2 and 3.

Breaking the pi bond between carbons 2 and 3 in but-2-ene
Then, the nascent oxygen unites with a hydronium (H+) from the ionization of water, forming hydroxyl groups (OH-), which bind to the free valences of carbons 2 and 3, resulting in a vicinal dialcohol.
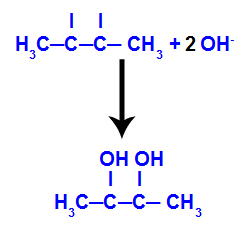
Vicinal alcohol formed from but-2-ene
→ Mild oxidation reaction on 2-methyl-propene
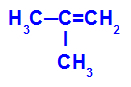
Structural formula of 2-methyl-propene
Initially, the double bond between carbons 1 and 2 of the alkene is broken by the attack of nascent oxygens, creating a free valence (vertical trace) on carbons 1 and 2.
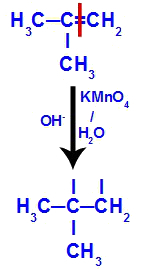
Breaking the pi bond between carbons 1 and 2 in 2-methyl-propene
Then, the nascent oxygen unites with a hydronium (H+) from the ionization of water, forming hydroxyl groups (OH-), which bind to the free valences of carbons 1 and 2, resulting in a vicinal dialcohol.
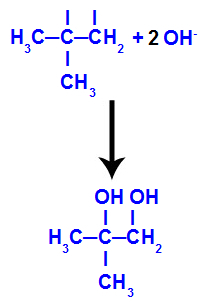
Vicinal alcohol formed from 2-methyl-propene
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidacao-branda-alcenos.htm