At intermolecular forces they are forces of electrostatic attraction whose function is to unite molecules (molecular compounds), keeping them in a solid or liquid state. They are extremely important, as they determine all physical properties (melting point, boiling point, density and solubility) of substances.
In this text we will study the relationship between intermolecular forces and the boiling point of substances. Initially, let's recall three important types of intermolecular forces, which are:
♦ dipole dipole: is the force that occurs in polar molecules. Since these molecules have a positive and negative pole, the dipole-dipole force is based on the attraction between the positive end of one molecule and the negative end of another. Examples: HCl, HBr, SO2 and PH3
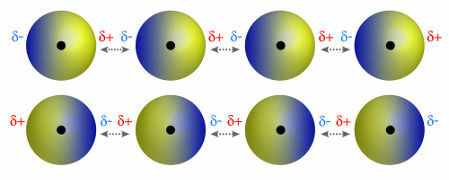
Molecules that have a positive and negative pole attracting each other
♦ Induced dipole: is the intermolecular force that occurs only in nonpolar molecules (they do not have poles). When two apolar molecules approach, a momentary deformation of their clouds occurs electrons, which causes an imbalance in the molecule's electrons, which are distributed in a different for her. At that moment, a momentary dipole is created, and the molecule momentarily has a positive and negative pole, which causes the attraction. Examples: CO
2, CH4 and BH3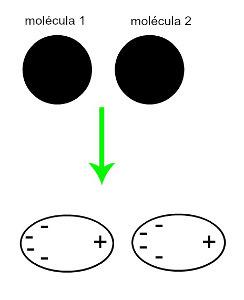
The approximation of two nonpolar molecules generates a deformation and, consequently, a redistribution of electrons, which forms the momentary dipoles
♦ Hydrogen Bonds: it is the intermolecular force that occurs in polar molecules, but only in those that mandatorily have hydrogen atoms bonded directly with fluorine, oxygen or nitrogen atoms. It can be considered a dipole-dipole force, but of much greater intensity. The interaction always takes place between the hydrogen of one molecule and the different atom (F, O, N) of another molecule. Examples: H2O, NH3 and HF
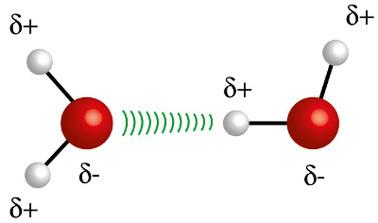
The hydrogen atom (white sphere) of one molecule interacts with the oxygen (red sphere) of another water molecule
Having remembered the three intermolecular forces, we can now relate them to the boiling point of substances. is called boiling point the temperature at which molecules of a given substance cease to be in a liquid state (have their intermolecular forces broken) and switch to a gaseous state. The interesting detail is that the intermolecular forces and the boiling point of substances have a very intense and direct relationship, since the more intense the intermolecular force, the higher the boiling point. The order of intensity of the intermolecular forces is:
Induced dipole < Dipole-dipole < Hydrogen bonds
Thus, we can conclude that molecules that have hydrogen bonds as an interaction force have higher boiling points than those that have dipole-dipole and so on. The table below shows three substances and their boiling point values:
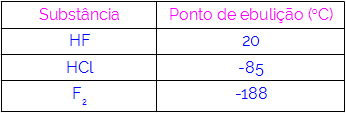
We observe in the table that HF has a higher boiling point, as its molecules are joined by hydrogen bonds. Substance F2 it has the lowest boiling point, since its molecules are attracted by an induced dipole.
By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/forcas-intermoleculares-ponto-ebulicao-das-substancias.htm
