For a long time, until the mid-nineteenth century, organic compounds were discovered and their names were given depending on their origin. For example, formic acid was first obtained through the distillation of red ants; urea was obtained through urine, lactic acid was acquired through milk, and so on.
However, over time, the amount of organic compounds discovered has increased and today it is recognized that there are more than 15 million of them. Thus, the need arose to formulate nomenclature rules for these compounds that could be applied internationally.
In addition, this nomenclature would have to achieve two specific objectives:
1) All compounds should have different names to distinguish them; there could not be two or more compounds with the same name;
2) It should be possible to name the compound by its structural formula and vice versa; that is, given the structural formula, it should be possible to elaborate its name.
In 1892, at the International Congress in Geneva, a discussion and rationalization began among chemists, in order to achieve a nomenclature that would meet these objectives. Several international meetings were then held and, finally, the so-called
IUPAC Nomenclature (International Union of Pure and Applied Chemistry, acronym that comes from English International Union of Pure at Applied Chemistry). Thus, this body was responsible for determining and drawing up the official nomenclature rules for all known organic compounds.Briefly, this nomenclature consists of three main parts:
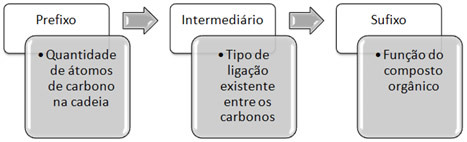
Note that compounds have been broken down into organic functions. Each role is characterized by a functional group. For example, if the compound has only carbons and hydrogens in its structure, it means that it belongs to the hydrocarbon group. If you have the OH group attached to a carbon, it constitutes an alcohol and so on. Compounds belonging to the same group have similar properties.
Below is a table specifying the terms most used in the nomenclature of organic compounds:
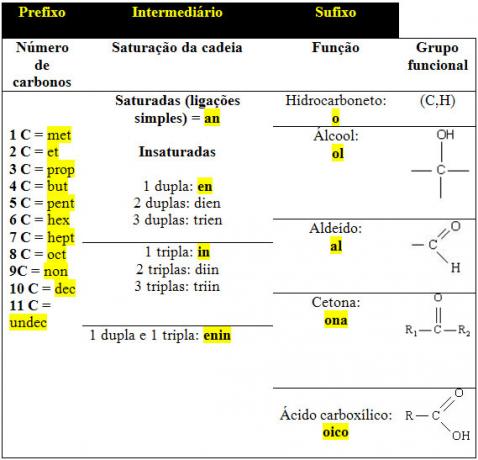
See some examples below:
H3CCH3:ethane
- Prefix: as it has two carbons, the prefix is et;
- Intermediate: only has single links: an;
- Suffix: as it only has C and H, it belongs to the hydrocarbon group: O.
O
║
H3CCCH3:propanone
- Prefix: has three carbons: prop;
- Intermediate: only has single bonds between carbons: an;
- Suffix: has a secondary carbon bond with an oxygen atom, so it is from the ketone group: whoa.
The IUPAC nomenclature is considered the official nomenclature for organic compounds. However, it did not totally eliminate other particular systems of nomenclature, such as the names cited at the beginning of this text. Thus, other forms of nomenclature of organic compounds that do not follow the rules of IUPAC are called usual nomenclatures.
Source: Brazil School - https://brasilescola.uol.com.br/quimica/nomenclatura-iupac.htm

