Carbonates are inorganic compounds formed by the ionic bonding of a metal or semimetal with the carbonate anion, CO32-.
Carbon is tetravalent, that is, it has four electrons in the valence shell, and it can make four covalent bonds to be stable, while oxygen is bivalent, having six electrons in the valence shell and being able to make two bonds to be stable, with eight electrons. Thus, there is a strong tendency for a carbon to bond to two oxygen atoms, all of which are stable (O ═ C ═ O → CO2).
But other oxygen can combine with carbon, since the ratio of ionic radii leads to a coordination number equal to 3, forming a structure triangular in which the carbon is in the center, making a double bond with one of the oxygen atoms and two single bonds with the other two oxygens. The result is two excess electrons, since these two oxygens are not stable, needing to receive one electron each:
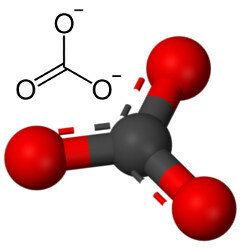
The carbonate anion is formed by covalent bonds, but its compounds, which are inorganic salts and minerals known as carbonates, are ionic, as this radical receives two electrons from some metal or semimetal, forming a ionic bond.
These compounds are insoluble in water, with the exception of ammonium carbonate ((NH4)2CO3) and carbonates formed with alkali metals (elements of family 1: Li, Na, K, Rb, Cs and Fr). Almost all are white solids, as shown in the image below:

The two most common and most important everyday examples of carbonates are sodium carbonate (Na2CO3) and calcium carbonate (CaCO3). In the first case, sodium belongs to family 1, having an electron in the valence shell and tending to lose this electron to become stable. As the carbonate anion needs to receive two electrons, it binds to two sodium atoms:

Soda ash is better known as soda or soda, and is used in the manufacture of soaps, dyes, medicines, paper and in the treatment of swimming pool water. But its main application is with calcium carbonate and sand in the manufacture of glass.
Calcium is family 2, having a tendency to lose two electrons. Thus, a calcium atom binds to a carbonate radical:

Calcium carbonate is present in limestone and marble. At stalactites and stalagmites that exist in caves are constituted of this carbonate; shells, coral reefs and eggshells too. When we whitewash walls, tree trunks and other places, we are using calcium hydroxide (Ca(OH)2), which over time reacts with atmospheric carbon dioxide to form calcium carbonate.

Carbonates are very common on the earth's surface, as in the case of minerals. Its crystalline reticulum can rearrange itself in space in two ways: the orthorhombic (as is the case with the mineral aragonite shown above together with the calcium carbonate formula) and the rhombohedral or trigonal, as is the case of calcite (another mineral consisting of calcium carbonate).
Carbonates react in the presence of acids, releasing CO2, which is easily seen through an effervescence.
By Jennifer Fogaça
Graduated in Chemistry