O vinyl chloride it is a hydrocarbon toxic chlorinated and carcinogenic action. It is a colorless gas at room temperature, being highly flammable and sensitive to heat.
It is the monomer used in the manufacture of polyvinyl chloride, a substance known as PVC. O PVC is a heat-resistant thermoplastic (unlike its monomer) that has numerous applications in the everyday life, such as coating electrical wires, construction of pipes and various types of packaging.
Read too:Asbestos — natural fiber with wide industrial application, but extremely toxic to humans
Summary on Vinyl Chloride
Vinyl chloride is a chlorinated hydrocarbon with the formula H2C=CHCl.
It is a gas colorless, sweet-smelling and highly flammable.
Vinyl chloride is unstable to heat, undergoing decomposition.
The main application of vinyl chloride is in the manufacture of polyvinyl chloride (PVC).
PVC is a thermoplastic with numerous applications, such as piping, parts, coatings, packaging, etc.
Vinyl chloride is toxic and has a carcinogenic effect.
vinyl chloride properties
molecular formula: CH2CHCl (C2H3Cl).
Molecular mass: 62.498 g/mol.
physical state: gas (colorless and with a strong odor).
Density: 0.91 g/ml.
Solubility in water: very slightly soluble (0.6g in 100 mL of water, 20 °C).
melting temperature: -154°C.
Boiling temperature: -13°C.
What is Vinyl Chloride?
Vinyl chloride is a chlorinated hydrocarbon with the formula H2C=CHCl. At room temperature, it occurs with a Colorless and highly flammable gas.
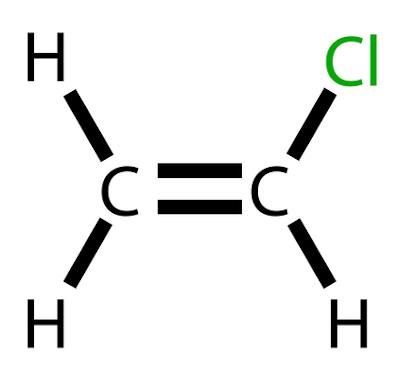
Vinyl chloride, also called chloroethene or vinyl chloride monomer, is a chemical product of great importance in industry, having special application in the manufacture of polymer polyvinyl chloride, better known as PVC. This compound occupies the list of the 20 products derived from Petroleum of greater industrial and economic relevance.
Read too:Benzopyrene — carcinogenic hydrocarbon found in cigarette smoke and grilled meats
Features of Vinyl Chloride
Vinyl chloride is a Colorless gas with a mild, sweet odor. It has the property of being highly flammable.
When exposed to heat sources, may undergo decomposition, emitting toxic vapors in carbon dioxide, carbon monoxide, hydrogen chloride and phosgene. Because it is an organic compound, its solubility in water is extremely low, on the other hand it is soluble in substances such as ethanol, benzene and carbon tetrachloride.
In the presence of moisture, vinyl chloride becomes corrosive and can attack the iron and the steel. It has the capacity to polymerize when exposed to heat in atmospheric air, through an exothermic reaction. This property derives many of its industrial applications.
Vinyl chloride demands attention because it is toxic and carcinogenic.
What is vinyl chloride used for?
Vinyl chloride is the monomer used to manufacture PVC polymer (polyvinyl chloride) and other chlorinated solvents.
PVC is a thermoplastic used in the manufacture of packaging, footwear, electrical connections and cables, pipes, windows, tubes and blood collection bags, garments, among countless others items.

O PVC is formed by the polymerization of vinyl chloride. In this chemical process, a large number of vinyl chloride molecules add to each other, forming a large chemical structure.
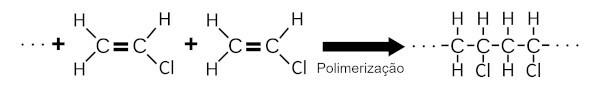
O PVC is flame retardant and for this reason it is widely used in coating wires, electrical cables and residential coatings.
Although vinyl chloride is a chemical compound that demands attention due to its toxicity and thermal instability, its PVC polymer is very stable to heat sources, it is non-toxic and can be stored with safety.
Until 1974, vinyl chloride was used in aerosols. In the past, it was even used as an inhalational anesthetic. With the knowledge of the toxicity of this compound, these applications were discontinued.
Obtaining Vinyl Chloride
The synthesis of vinyl chloride used on an industrial scale is initiated with the compound ethylene or ethene (CH2=CH2) and can occur by two routes.
In the first one, ethylene is converted into 1,2-dichloroethane through reaction with chlorine gas. Then, by heating the 1,2-dichloroethane in the presence of a catalyst, vinyl chloride is obtained as the main product and the hydrochloric acid as a secondary product.
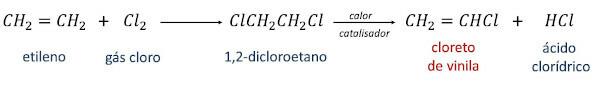
In the second reaction route, known as oxychlorination, the reaction between ethylene, hydrochloric acid and oxygen from the atmosphere itself, in the presence of heat and a catalyst, generating vinyl chloride and water as products.

Typically, a vinyl chloride manufacturing center is built to accommodate both manufacturing processes. cited, so that the hydrochloric acid generated in the first method acts as a reagent for carrying out the second route.
Precautions with Vinyl Chloride
Vinyl chloride is a toxic compound. Since it is a gas, The main form of contamination is by inhalation., which is why its handling must always be done with the use of suitable equipment, such as gas masks.
Exposure to this substance affects the nervous system peripheral and central, causing damage to the liver. A Continuous exposure can trigger Raynaud's phenomenon, which is a set of symptoms that include joint and muscle pain and skin changes, which can progress to a complete loss of skin elasticity, even affecting organs internal organs and blood vessels.
Other effects include euphoria, disorientation, miscarriage and birth defects. Injuries to ocular tissue are also recorded.
Symptoms depend on the level of exposure to the substance, ranging from dizziness, nausea, visual disturbances, headache and ataxia in acute exposure (from 1000 to 8000 ppm vinyl chloride in air), to narcotic effect, cardiac arrhythmia and fatal respiratory failure in cases of exposure to levels above 12000 ppm.
Vinyl Chloride and Cancer
O vinyl chloride is a carcinogenic substance, associated with a high risk of developing liver cancer, which may contribute to occurrences of brain cancer and lung, as well as cancer of the lymphatic system.
Read too:Ammonium nitrate — compound used in agriculture that can cause explosions
Occurrence of Vinyl Chloride
the vinyl chloride is generated spontaneously in the environment through the decomposition of some compounds containing chlorinethrough the action of microorganisms. Therefore, it can be considered an air and water contaminant, especially in regions close to landfills.
However, the highest occurrence of vinyl chloride is by synthetic routes, related to the chemical industry, as previously discussed.
History of Vinyl Chloride
Vinyl chloride was discovered in 1835 by the German chemist Justus von Liebig, when reacting dichloroethane with potassium hydroxide in an alcoholic environment.
Later, in 1872, the chemist Eugen Baumann observed for the first time the polymerization of vinyl chloride, originating PVC, after leaving a container with the substance exposed to the Sun by accident.
In 1926, the American inventor Waldo Semon discovered chemical additives that provided greater elasticity and malleability to PVC, expanding the application possibilities of this substance. Around 1950, PVC began to be used on an industrial scale. Currently, PVC is one of the most used thermoplastics in the world.
By Ana Luiza Lorenzen Lima
Chemistry teacher
Source: Brazil School - https://brasilescola.uol.com.br/quimica/cloreto-de-vinila.htm
