O muscovius, atomic number 115, located in group 15 of the Periodic Table, is one of the last elements included in it, in 2015, along with elements 113, 117 and 118. Its name is a reference to the region of Moscow, Russian capital.
Moscovium, however, was initially produced, in 2003, through the joint work of Russian and American scientists. Even so, nearly 20 years after its initial synthesis, its basic properties are still being determined. Thus, much is speculated and little is known about its properties.
Know more: Names of the new chemical elements — the tributes to cities, regions and scientists
abstract about moscovius
It is a synthetic chemical element located in group 15 of Periodic table.
It was synthesized for the first time, in 2003, through joint work between Russian and American scientists.
It makes up the group of elements most recently included in the Periodic Table, in 2015.
Their studies are very recent, with basic properties still being determined.
Its production takes place by nuclear fusion, using 48Ca and atoms of 243Am.
Moscow properties
Symbol: Mc
Atomic number: 115
Atomic mass: 288 au.m.a (not official by Iupac)
Electronic configuration: [Rn] 7s2 5f14 6d10 7p3
Most stable isotope: 288Mc (0.159 second half-life)
chemical series: group 15, superheavy elements
Muscovy features
the muscovius is one of the last elements includeds in the Periodic Table. Its inclusion took place on December 30, 2015, with its official name being released on June 8, 2016.
Until that date, element 115 was known in Portuguese as ununpentio, from the Latin, ununpentium, whose translation is “one, one, five”. Another nomenclature adopted was eka-bismuth, which means "similar to bismuth", element of the sixth period of group 15.
The Muscovy is a synthetic element, which means that it can only be produced in a laboratory. This is very common among superheavy elements because their nucleus, with many protons and neutrons, cannot stabilize, making it impossible to find them in nature.
for being a unstable element, it and the other superheavy elements end up undergoing radioactive decay almost instantly — particle emissions nuclear elements (such as α or β particles) — and the consequent transformation into other lighter elements, which may be stable or no.
With regard to it, it should be noted that its studies are still very recent, after all, we are facing an element produced just under 20 years ago and whose official status is not even 10 years old. In this regard, scientists have been more concerned with determining basic characteristics, such as their atomic mass and its chemical behavior in some possible compounds.
For example, the most likely atomic mass detected so far for muscovy is 288 atomic mass units. Not to mention that obtaining the muscovium is very complicated, with a income from just one atom per day.
In addition, the produced atom cannot always be captured to measure the mass. In 2018, researchers at Berkeley Laboratories, California, United States, were able to measure just one mass per week. Thus, studies about the properties of its compounds are still in the field of theoretical chemistry, with calculations and mathematical models for determining the expected results.
Obtaining the Muscovy
Obtaining the moscovium is done by Nuclear fusion. ions of 48Here11+ (Z = 20) accelerated hit atoms of 243Am (Z = 95), arranged in the form of AmO2 on a circular target titanium of 32 cm², producing the moscovium (Z = 115) and three neutrons.
After impact, in about a microsecond (10-6 second), the muscovy atom hits the detector, which is about four meters away from the collision site. On this path, the element also passes through a separator, so that lighter reaction products are diverted. In the detector, the muscovium is detected by its radioactive decay pattern.
Moscovium, as a radioactive atom, undergoes alpha decay (a radioactive particle with two protons and two neutrons), thus producing element 113 (nihonium, Nh) to element 105 (dubnium, Db). Finally, the Db turns into the rutherfordium (Rf), which splits into two fragments quickly. The decay pattern of moscovium is shown below.
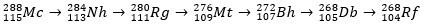
history of moscow
the muscovius was first synthesized in 2003, between July 14th and August 10th, through the joint work of scientists from the Joint Institute for Nuclear Research in Dubna, Russia, and the Lawrence Livermore National Laboratory in Livermore, California.
Ions of 48Ca so that they could collide with atoms of 243Am, initially producing the isotope 291Mc. During the process, the core was heated to an incredible 4 x 1011 K, and then cooled by the very rapid emission of three neutrons and gamma rays.

This action formed the isotope 288Mc. then the moscovius was detected and analyzed based on its pattern of radioactive decays (alpha decays). The name Moskow is a tribute to the Moscow region, Russia.
Read too:Seaborgium — the synthetic chemical element named after scientist Glenn Seaborg
Solved exercises on muscovius
question 1
Moscovium, a recently discovered element, was placed in group 15 of the Periodic Table. Based on the other elements in this group, the expected hydride for this element would be:
A) McH
B) McH2
C) McH3
D) McH4
E) Mc2H3
Resolution:
Alternative C
Other group 15 elements, such as nitrogen It is phosphor, present the formulas NH3 and pH3 when bonded to hydrogen. Thus, it is expected that the moscovium presents the formula McH3 also.
question 2
In 2003, moscovium (Z = 115) was synthesized for the first time, through the joint work of Russian and American scientists. At the time, the isotope 288Mc was detected and its production was essential to place this element in the Periodic Table. The number of neutrons in this isotope is:
A) 115
B) 288
C) 403
D) 173
E) 170
Resolution:
Alternative D
The number of neutrons can be calculated like this:
A = Z + n
Where A is the mass number, Z is the atomic number, and n is the number of neutrons. Substituting the values, we get:
288 = 115 + n
n = 288 – 115
n = 173
By Stefano Araujo Novais
Chemistry teacher
