THE Law of Speed of Reaction relates the speed of a chemical transformation with the concentrations of reagents in quantity of matter (mol/L), which can be stated as follows:

For example, consider the following generic reaction:
aA + bB → cC + dD
Let's say we increase the concentration of reactants A and B, what will happen to the reaction rate? Well, as the quantity of reactant particles will increase in the same space, there will be more effective collisions between them, which will result in an increase in the rate of reaction development. Which means it will increase your speed.
Therefore, the reaction rate is directly proportional to the concentration of the reactants. However, it depends on the temperature as well. Therefore, we have the following mathematical equation that represents the law of reaction speed:

On what:
v = reaction speed;
k = constant that only depends on the temperature value;
α and β = exponents determined experimentally.
Only when the reaction is elementary, that is, it occurs in a single step, are the exponents exactly equal to the coefficients of the balanced chemical equation:
v = k. [THE]The. [B]B. However, in other cases, the appropriate potency to which the concentration of each reagent must be raised must be determined experimentally.The law of speed of reactions goes by many names, here are some: Law of Mass Action, Equation of Rapidity, Kinetic Law of Reaction and Guldberg-Waage Law.
Consider an example of how to apply this law:
Consider the following elementary reaction:
2 HCl (g) → H2(g) + Cl2(g)
a) Write the equation for the speed of this reaction;
b) Through experiments, the speed of this hydrogen chloride gas decomposition reaction and the concentration of this reagent, at a constant temperature of 25 °C, were noted in the table below:
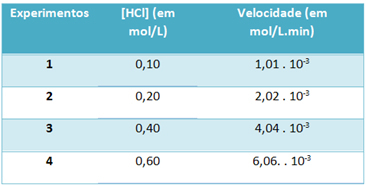
Based on this, determine the characteristic velocity constant of this reaction at the mentioned temperature.
Resolution:
The) v = k. [HCl]2
b) v = k. [HCl]2
k = __v___
[HCl]2
k = 1,01. 10-3 mol. L-1. min-1
0.01 mol. L-1
k = 1.01. 10-1 min-1
To solve the letter “b”, you can use the data of any of the experiments that the obtained value will be the same.
But what if the reaction isn't elementary? How will it be possible to resolve questions like these in non-elementary reactions? To find out how, read the Law of velocity for non-elementary reactions.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/lei-velocidade-das-reacoes-quimicas.htm
