Meet Yara! The artificial intelligence of Brasil Escola! It corrects essays in the Enem standard and answers questions about different disciplines quickly and free of charge!
A nomenclature of hydrocarbons is mainly characterized by the presence of the suffix “-o”. Such nomenclature rules are defined by the International Union of Pure and Applied Chemistry, through a book popularly known as “The Blue Book”.
The hydrocarbons (organic function that has only carbon and hydrogen in its structure), however, vary the infix, as they can be saturated (like alkanes and cycloalkanes) and unsaturated (like alkenes, alkynes and cycloalkenes). Aromatics (such as benzene) also have a specific system of nomenclature, not much different, however, from closed chains.
Read too: How to know the nomenclature of compounds with mixed functions?
Topics of this article
- 1 - Summary on the nomenclature of hydrocarbons
- 2 - Video class on nomenclature of hydrocarbons
- 3 - What is the naming rule for hydrocarbons?
- 4 - Nomenclature of alkanes
- 5 - Nomenclature of alkenes
- 6 - Nomenclature of alkadienes
- 7 - Nomenclature of alkynes
- 8 - Nomenclature of cycloalkanes
- 9 - Nomenclature of cycloalkenes
- 10 - Nomenclature of aromatics
- 11 - Solved exercises on nomenclature of hydrocarbons
Summary on the nomenclature of hydrocarbons
All hydrocarbons have the suffix “-o”.
The naming rules are defined by the International Union of Pure and Applied Chemistry, IUPAC.
Although they do not vary in the suffix, the hydrocarbons will vary in the infix, being “-an-” for those that have saturated chain, “-en-” for those with a double bond and “-in” for those with a double bond triple.
Aromatics, such as benzene, have their own nomenclature system, a little different from the others. hydrocarbons, however with similarities in relation to the nomenclature system of chain compounds closed.
Video lesson on nomenclature of hydrocarbons
What is the naming rule for hydrocarbons?
Hydrocarbons, as well as all other compounds of Organic Chemistry, have their official (or systematic) names determined by IUPAC (in Portuguese, International Union of Chemistry).
Such regulations are updated from time to time and are included in the book Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names, whose free translation can be Organic Chemistry Nomenclature: IUPAC Recommendations and Preferred Names. Such a book is commonly called “The Blue Book” of IUPAC.
Hydrocarbons, according to current regulations, must always have the suffix “-o”.
Do not stop now... There's more after the publicity ;)
nomenclature of alkanes
the alkanes are hydrocarbons that have an open and saturated chain. Consequently, have, in addition to the suffix “-o” of hydrocarbons, the infix “-an-”, indicating only single bonds between carbon atoms.
Examples:
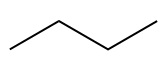
The prefix “but-” is used to indicate 4 carbons in the chain.
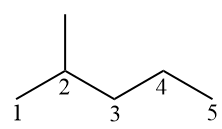
If there are branches in the alkane, these must have as few branches as possible.. Thus, the main chain (pentane) must begin its numbering from the leftmost end, so that the methyl has the lowest possible number (2).
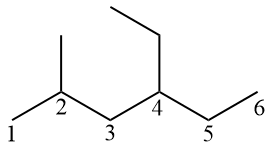
The main chain must be numbered from left to right, so that the branches are at carbons 2 and 4. If numbered from right to left, the branches would be on carbons 3 and 5, which would be longer.
Although methyl receives the lowest number, in the official nomenclature the branches (or radicals) must be in alphabetical order. Therefore, ethyl (which starts with E) comes before methyl (which starts with M). By the Portuguese language, a hyphen must be used before words that begin with the letter H. Thus, we use a hyphen in “methylhexane” but not in “methylpentane”.
See too: What is the nomenclature of alkanes with more than ten carbons?
nomenclature of alkenes
The alkenes are hydrocarbons that also have an open chain, but have a double bond between carbon atoms, which makes them unsaturated. This brings about a change in the name in relation to alkanes, which is the replacing the infix "-an-" of alkanes with "-en-". In addition, double bonds, according to Iupac, must also be numbered. Double bonds should also be as few as possible and have priority over branches.
Examples:
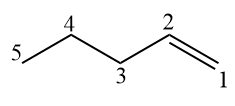
For alkenes with more than 3 carbons, the double bond must be numbered next to the “-en-” infix in the official name.
Between a branch and a double bond, priority is given to the double bond to have as few as possible.
nomenclature of alkadienes
The Alcadienes are hydrocarbons that have two double bonds. The infix remains “-en-”, but with the addition of the numerical descriptor “di-” preceding “-en-” to indicate that there are two double bonds. In terms of phonetics, the letter “a” is added after the main string prefix.
Example:
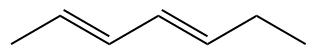
nomenclature of alkynes
the alkynes are hydrocarbons that have the same particularities as the rules of alkenes, with the difference that they have a triple bond instead of a double bond. This also brings a difference in the infix, with replacing the infix “-en-” with “-in-”.
Examples:
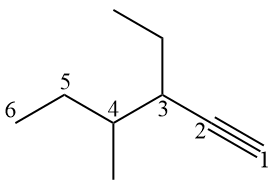
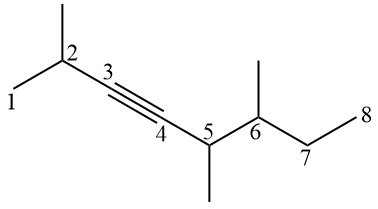
Since the sp carbon has a linear geometry, it is common to represent the alkyne with a linear geometry in the triple bond, making it difficult to count the carbons at first. The idea is to visualize the π bonds, which limit the carbons present there.
Nomenclature of cycloalkanes
The cycloalkanes are hydrocarbons that have a closed chain and are saturated. Therefore, in its official name, will have the prefix “ciclo-” before the name of the main chain, as well as the infix “-an-”, traditional saturated chains.
Examples:

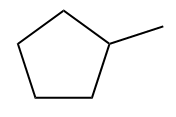
Monosubstituted cycloalkanes (with a branch) must not have numbering for the branch in the official name, as it is redundant (after all, the branch must be in position 1).
However, if there are more than two branches, these must be numbered normally in the official name, with number 1 prioritized in terms of alphabetical order. Then, the numbering must rotate clockwise or counterclockwise so that the other branches have the lowest number possible.
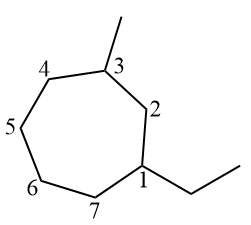
Note that the ethyl branch has the number 1, as the letter E comes before the letter M, for methyl, in the alphabet. Afterwards, the numbering of the cycle rotated counterclockwise, so that the methyl branch had the lowest possible number (3).
Nomenclature of cycloalkenes
Cycloalkenes are hydrocarbons that have an unsaturated chain and, therefore, have the infix “-en-”. Being branched, priority will be given to unsaturation, just like alkenes.
Examples:
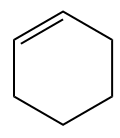
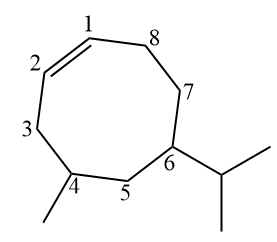
In the case of the previous branched structure, carbons 1 and 2 will always be those of the double, but they will be numbered so that the branches have the lowest possible number. Isopropyl, however, is ahead of methyl in terms of alphabetical order and is therefore written first (I comes before M).
Nomenclature of aromatics
The aromatic hydrocarbons have structures that have obligatorily cycle or hexagonal cycles containing three alternating double bonds. In high school, a good part of the study of aromatic hydrocarbons is retained benzene (C6H6). Benzene follows the IUPAC recommendations for closed-chain hydrocarbons, but the name “benzene” is accepted for the main chain.
For disubstituted benzene compounds, Iupac no longer officially recommends the use of the ortho location descriptors (o), meta (m) and para (p), however such locators are still widely used in tests and contests and therefore will be cited here.
Examples:

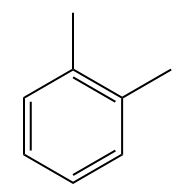
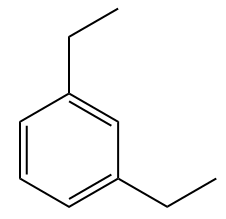
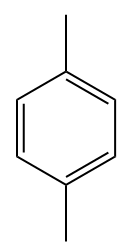
Naphthalene, which consists of two condensed benzene rings, has a fixed number, according to IUPAC:
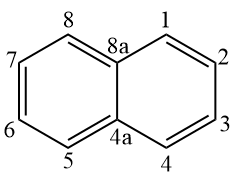
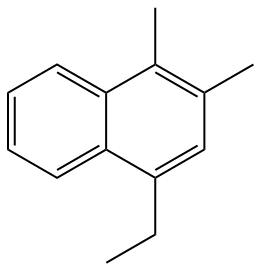
Therefore, the following structure must be named according to the fixed numbering.
Know also: What are the main organic functions?
Solved exercises on hydrocarbon nomenclature
question 1
(IME) Isoprene is a toxic organic compound that is used as a monomer for the synthesis of elastomers, through polymerization reactions. Given the structure of isoprene, what is its Iupac nomenclature?

A) 1,3-butene
B) 2-methylbutadiene
C) 2-methylbutene
D) pentadiene
E) 3-methyl-butadiene
Resolution:
Alternative B.
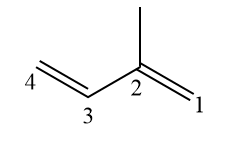
The numbering for the structure is represented in the previous image. With the branching at carbon 2 (branches should have the smallest possible number), unsaturation can only be at carbons 1 and 3, with no other possible position. Therefore, they are omitted from the official name, as it is redundant to say buta-1,3-diene.
Therefore, the name remains as 2-methylbutadiene.
question 2
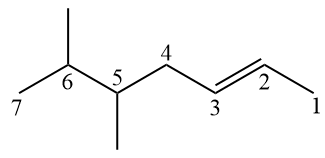
(UEG) The hydrocarbon below, according to the IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules, is the
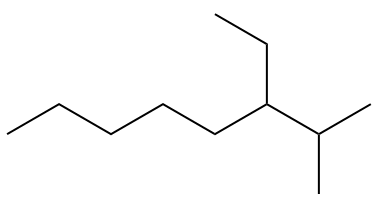
A) 3-ethyl-2-methyloctane.
B) 6-ethyl-7-methyloctane.
C) 3-isopropyloctane.
D) 2-methyl-3-ethyloctane.
Resolution:
Alternative A.
Note the numbering for the hydrocarbon in question in the image below.
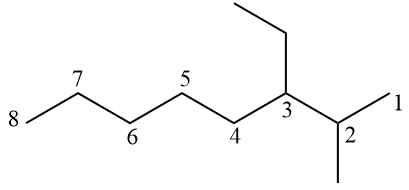
Branches should be as few as possible, so numbering starts from the far right. When writing the official name, the branches must be placed in alphabetical order: 3-ethyl-2-methyloctane.
Source
FAVRE, H. A.; POWELL, W. H.; MOSS, G. P. Nomenclature of Organic Chemistry. IUPAC Recommendations and Preferred Names 2013. London: Royal Society of Chemistry, 2013.
By Stefano Araujo Novais
Chemistry teacher
Here in this text you will find the definition of the functional group of alkadienes, you will see some examples of them in everyday life and how their nomenclature is carried out.
Learn more about alkanes, as well as their physicochemical properties, official nomenclature, general formula and applications. Do exercises on the topic.
Click here, find out what alkenes are, learn about their properties and characteristics and understand the criteria used in their official nomenclature.
Cycloalkanes are cyclic and saturated hydrocarbons, that is, with a closed chain and with single bonds.
Aromatic compounds, arenes, polarity, insoluble, soluble, nonpolar solvents, ether, carbon tetrachloride, hydrocarbons, insecticides, dyes, solvents, explosives, carcinogenic, toluene, methylbenzene, drugs, glue shoemaker.
Learn more about the properties, types, nomenclature and where hydrocarbons can be found.