A sublimation It is a change in the physical state of matter characterized by the direct passage from the solid phase to the gaseous phase, without passing through the liquid phase. Strictly speaking, any substance can undergo sublimation, but under specific conditions of pressure and temperature. Sublimation is correlated with the vapor pressure in the solid phase, as well as with the intermolecular interactions exerted by the solids.
This process can be easily observed in a piece of dry ice, which consists of solid carbon dioxide. Carbon dioxide sublimates under pressure and at room temperature. The opposite process to sublimation can be called resublimation or deposition. For sublimation to occur, matter must absorb energy, so it is considered an endothermic process.
Read too: What are the physical states of matter?
Topics of this article
- 1 - Summary on sublimation
- 2 - What is sublimation?
-
3 - Operation of sublimation
- What is vapor pressure?
- Vapor pressure and sublimation
- Phases diagram
- 4 - Examples of sublimation
- 5 - Solved exercises on sublimation
Summary about sublimation
Sublimation is the direct passage from the solid phase to the gaseous phase, without going through the liquid phase.
Specific conditions of pressure and temperature are necessary to observe the sublimation of a substance.
Sublimation is influenced by thermodynamic aspects, such as vapor pressure in the solid phase and intermolecular interactions.
Sublimation is an endothermic process.
An example of sublimation is what occurs in dry ice, which consists of solid carbon dioxide.
What is sublimation?
sublimation is the direct transition from the solid phase to the gas phase, without going through the liquid phase. It occurs under specific conditions of temperature and pressure for some solids. This transition is a physical process of state change, not involving chemical reactions.
The reverse process, i.e. the direct passage from the gaseous phase to the solid phase, is variously called. Some authors keep the word sublimation for this phase change, while others use “resublimation” and even “deposition”.
Do not stop now... There's more after the publicity ;)
How sublimation works
A parallel can be drawn between sublimation and evaporation. In both cases, the endpoint is gas phase. The difference, obviously, is in the starting phase: solid for sublimation and liquid for evaporation.
In both cases, there is influence of pressure devapor and also thermodynamic aspects, involving heat and intermolecular interactions.
What is vapor pressure?
In a closed container containing a liquid, it is possible to perceive that there is an equilibrium between the liquid phase and the vapor phase. This occurs because, even below the boiling temperature, the energy present is sufficient for some molecules of the liquid to detach and pass into the vapor. However, some vapor molecules can also condense again and return to the liquid phase, which demonstrates the reversibility of the process.
This vapour, being made of matter (has mass and volume), exercises pressure on the surface of the liquid, known as vapor pressure. It is dependent not on the amount of liquid, but on the temperature, because the higher the temperature, the more easily the molecules are detached from the liquid phase.
Liquids that have a high vapor pressure at ordinary temperatures are called volatile. For example, at 25°C, ethyl ether has a vapor pressure of 0.58 atm, acetone (propanone) has a vapor pressure of 0.29 atm, while water has a vapor pressure of 0.023 atm. By the way, when the vapor pressure is identical to the atmospheric pressure, the liquid boils. To learn more about vapor pressure, click here.
Vapor pressure and sublimation
Although to a lesser extent, solids also have vapor pressure, but much lower than that of liquids. For example, even at a temperature of 1000 K, the vapor pressure of iron is only 9.21 x 10-20 atm. However, some solids manage to undergo sublimation, such as iodine, presenting a higher vapor pressure at normal temperatures (4 x 10-4 atm).
This is possible only with the passage of molecules from the solid state directly to the gaseous state. For this to happen, the molecules of the solid must present intermolecular interactions weak (in iodine, for example, they are of the induced dipole-induced dipole type).
It is also seen that the sublimation process is endothermic, that is, there is a need for the molecules of the solid to absorb energy in the form of heat so that they can break the intermolecular interactions and pass to the vapor state. The amount of heat involved can be measured by a thermodynamic quantity known as the enthalpy of sublimation.
Phases diagram
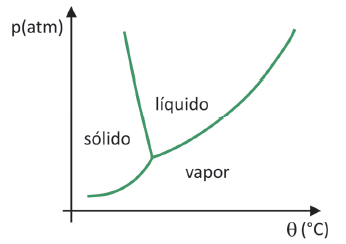
To to know in which range of pressure and temperature the sublimation of a solid will occur, you need to evaluate your phase diagram. Let's look at the case of carbon dioxide, CO2.

In a phase diagram, the boundary lines between states (solid, liquid, and gas) bring together the pressure and temperature values for a change of state occurs. When observing the case of CO2, it is noticed that at 1 atmosphere of pressure, the solid phase passes directly to the vapor phase at a temperature of -78.5 °C, which characterizes a sublimation.
Carbon dioxide only has a liquid phase at pressures above 5.11 atmospheres, and beyond that pressure, sublimation is no longer possible. To learn more about the phase diagram, click here.
examples of sublimation
Dry ice: dry ice, often used to make fog effects at parties and events, is actually carbon dioxide in solid state.

mothballs: mothballs are made from naphthalene, an aromatic organic compound. It is applied to remove bad odors and also scare away moths, cockroaches and other venomous animals, which is why it is common for them to be used in cabinets or even urinals.

Camphor: With a characteristic odor, camphor pebbles can also undergo sublimation. They also serve to scare away mosquitoes and prevent mold.

Iodine: the non-metal belonging to the halogens also undergoes sublimation.

However, among the substances presented, only carbon dioxide undergoes sublimation under ambient conditions. The others, even with sublimation, can undergo normal fusion under the pressure we live in.
Read too: Plasma — the fourth state of matter
Solved exercises on sublimation
question 1
(Fuvest 2020) In supermarkets, it is common to find so-called freeze-dried foods, such as fruits, vegetables and meat. Freeze-dried foods are still suitable for consumption after a long time, even without refrigeration. The term “lyophilized” in these foods refers to the freezing process and subsequent dehydration by water sublimation. For sublimation of water to occur, a combination of conditions is required, as shown in the pressure-temperature graph, where the lines represent phase transitions.
Despite being a process that requires, industrially, the use of certain technology, there is evidence that peoples pre-Columbians who lived in the highest regions of the Andes were able to freeze-dry food, making it possible to store it for more time. Check the alternative that explains how the natural freeze-drying process occurred:
a) Sublimation of water occurred due to low temperatures and high atmospheric pressure in the mountains.
b) Food, after being naturally frozen in cold periods, was taken to the lowest part of the mountains, where the atmospheric pressure was lower, which made sublimation possible.
c) The food was exposed to the sun to increase the temperature, and the low local atmospheric pressure favored solidification.
d) Temperatures were low enough in the cold periods to freeze food, and the low atmospheric pressure in the high mountains made sublimation possible.
e) The foods, after being naturally frozen, were pressed to increase the pressure, so that sublimation could occur.
Response: Letter D.
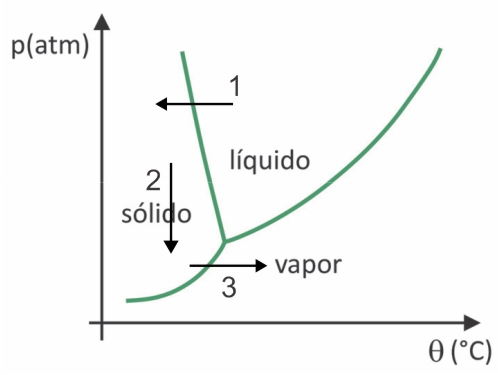
In lyophilization, there is the freezing of water with its subsequent sublimation. Pre-Columbian peoples could carry out such a process, as in winter the water could freeze (arrow 1) and, with the low pressures (arrow 2) of the highest regions of the Andes, it could undergo sublimation (arrow 3).
question 2
(Uerj 2005) Dry ice, or solidified carbon dioxide, widely used in refrigeration processes, undergoes sublimation under ambient conditions. During this transformation, the phenomena of energy variation and disruption of interactions occur, among others.
These phenomena are classified, respectively, as:
a) exothermic - interionic
b) exothermic - internuclear
c) isothermal - interatomic
d) endothermic - intermolecular
Response: Letter D.
Sublimation is an endothermic process, as it requires absorption of heat to disrupt the interactions that keep the solid phase molecules compact. These interactions are of the intermolecular type.
By Stefano Araujo Novais
Chemistry teacher
Learn more deeply about this phenomenon.
The answer lies in pressure and temperature changes.
Learn about the physical states of matter and see what they are. Also learn what Bose-Einstein is and where we can find matter in the plasma state.
Induced dipole-induced dipole forces are the weakest intermolecular forces that occur between nonpolar molecules.
See how to identify if a substance has a higher boiling point than another by the relationship between intermolecular forces and boiling point.



