THE fractional distillation is a process used to separate homogeneous mixtures formed by at least two miscible liquids. Separation by this method is based on the different boiling points of the components of the mixture.
This type of separation is generally applied to mixtures that have components with a boiling point difference of about 25 °C.
The main apparatus for the process is the fractionation column, also called a distillation column, which has a series of obstacles inside to prevent all components of the mixture from volatilizing at the same time.
Basically, the mixture is heated in a distillation flask until the physical state of the component with the lowest boiling point changes. Then, the steam passes through a fractional column, which separates the fractions, and after that the condenser makes the substance return to the liquid state so that it is directed to the collection container.
Fractional distillation is widely used in industries and laboratories, having great commercial significance. Examples of applications are: water purification, separation of petroleum components and separation of liquefied air.
Petroleum, for example, is a mixture of hydrocarbons, such as butane (PE 20 °C), gasoline (PE 150 °C) and kerosene (PE 300 °C). Therefore, the order of separation of the components in fractional distillation from the lowest to the highest boiling point is: butane, gasoline and kerosene.
Fractional distillation equipment
In fractional distillation, a system composed of the following parts is used:
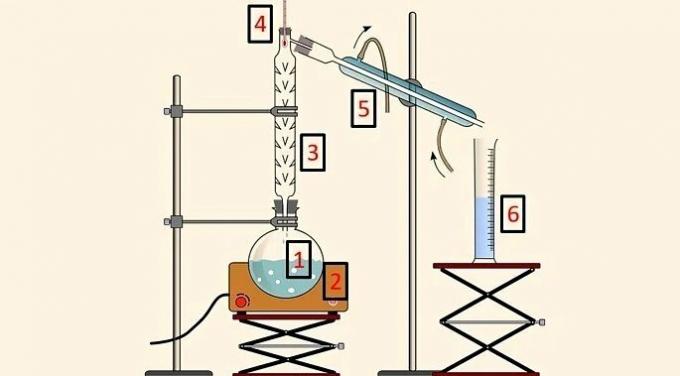
- Distillation flask (1), which contains the mixture;
- Heating blanket (2), which provides heat to the mixture;
- Distillation column (3), which allows separation according to temperature;
- Thermometer (4), to follow the temperature change;
- Condenser (5), to cool the evaporated component;
- Beaker (6), to collect the separated component in the liquid state.
Separation process in fractional distillation
First, the mixture is placed in the distillation flask together with the anti-collision granules to prevent rapid evaporation.
The fractionated column is connected to the distillation flask. As the mixture is heated, steam rises into the column, the interior of which is lined with "obstacles", usually of glass or porcelain beads.
The contact of the fluid inside the column causes the substances of the mixture to lose heat when passing through the equipment and only the component with the lowest boiling point goes to the top of the column.
The vapor that reaches the condenser is then cooled until it liquefies, that is, it returns to the liquid state and is collected in the receiving flask.
Read too: What is distillation?
Fractional distillation of petroleum
Oil is a complex mixture of organic compounds and the different components separated in the distillation process are called fractions. The fractions of petroleum are formed by hydrocarbons, such as gasoline, diesel, kerosene and bitumen.
Fractions are separated in the distillation tower, a steel column filled with trays that have "obstacles" for the passage of oil.
Initially, the oil is heated in a furnace and the heated mixture is fed to the bottom of a distillation tower. The tower temperature is higher at the bottom and lower at the top to prevent the heavier fractions from evaporating with the lighter components.
The longer carbon chain hydrocarbons are condensed and removed at the bottom of the tower. These fractions are dark, viscous and more difficult to evaporate. The single-chain ones, on the other hand, have lower boiling points and, therefore, condense at temperatures closer to the top of the tower, where they are collected.
know more about oil refining.
Difference between simple and fractional distillation
Simple distillation is applied when we want to separate a solid dissolved in a liquid. For this, we heat the mixture until the solvent evaporates. This process is faster and requires less energy than fractional distillation.
An example of a mixture that can be separated by simple distillation is water and salt. The water, having a boiling point much lower than table salt, is evaporated when the mixture is heated and, after passing through a condenser, it is collected in another container.
Fractional distillation is applied to the separation of liquids that are miscible with each other, such as water and acetone.
Gain more knowledge with the contents:
- Simple and fractional distillation
- Mixture separation
- Mixture separation exercises
Bibliographic references
BROWN, T. L.; LEMAY JR., H. AND.; BURSTEN, B. AND.; BURDGE, J. R. Chemistry the core science. 9th ed. Pearson Prentice Hall do Brasil, 2008.
USBERCO, J.; SALVADOR, E. General chemistry. 12th ed. Sao Paulo: Saraiva, 2006.
RUSSELL J B. General chemistry. vol. 1. Makron, 1996.
- Distillation
- Simple and Fractional Distillation
- oil refining
- Mixture separation exercises
- Separation of Mixtures
- Materials used in the Chemistry laboratory
- laboratory glassware
- Exercises on Organic Chemistry with Answers