THE teneso (or tennesso), symbol Ts, is the element of number atomic 117 of the Periodic Table. Its discovery was very recent, in 2009, with its inclusion in the Periodic Table only at the end of 2015. It is not found in nature in the form of any isotope and, therefore, must be produced in the laboratory, being therefore a synthetic chemical element.
Tennesso's properties are still being studied through theoretical chemistry and mathematical calculations, given its low production rate. Its production takes place through the reaction between the 48Ca and the 249Bk, being possible to produce the 294 or 293 isotope of the element.
The name refers to the US state of Tennessee, place of origin of some scientists involved in the discovery and production of the isotope. 249Bk, so important for the synthesis of this new element.
See too: Bohrium — another synthetic chemical element that has a low rate of production
tennesso summary
Tenesso is a synthetic chemical element located in group 17 of the Periodic table.
It was first synthesized in 2009, in a joint work between Russian and American scientists.
It was independently confirmed by German scientists.
It makes up the group of elements most recently included in the Periodic Table, in 2016.
Their studies are still very recent, and their properties are being stipulated by mathematical methods.
Its production is Nuclear fusion, using ions of 48Ca and atoms of 249bk.
Its name refers to the US state of Tennessee.
tennesso properties
Symbol: Ts.
Atomic number: 117.
Atomic mass: 293 c.u. or 294 c.u. (not official by Iupac).
Electronic configuration: [Rn] 7s2 5f14 6d10 7p5.
Most stable isotope:294Ts (51 milliseconds of half life, which can vary by 38 milliseconds more or 16 milliseconds less).
Chemical Series: group 17, halogens, superheavy elements.
Tenesso features
The tennesso (or tennesso), symbol Ts, was one of the last four elements to be made official by the International Union of Pure and Applied Chemistry (IUPAC) in its Periodic Table. With atomic number 117, it is located in group 17 of the halogens.
It was first produced between the years 2009 and 2010, but its confirmation by Iupac only took place on December 30, 2015. Elements of this size of atomic number and number of neutrons are not found in nature, and must be created in the laboratory, so it is a synthetic chemical element.
The main reason they are not found in nature is that they are extremely unstable. Once produced through nuclear reactions, they undergo radioactive decay in a few seconds (sometimes less than that, in the millisecond range).
In addition, elements such as Ts are produced slowly, with low yield. Specifically in the case of tenesso, the researchers kept the reaction for 70 days in order to detect six atoms of this element.
Therefore, at this time, researchers are trying to determine basic properties of Ts and some of its compounds through theoretical calculations and mathematical models. In a study carried out and published in Chemical Physics Letters, Brazilian researcher Robson Fernandes de Farias estimated some physical properties of Ts and tennesso, TsH, such as covalent radius, polarizability, covalent bond distance, as well as bond energy covalent.
Know more: Oganessone — the chemical element with the highest atomic number in the Periodic Table
obtaining teneso
Superheavy elements such as teneso are obtained by a technique called hot fusion reaction (free translation of hot fusion reaction). In this technique, it is common to use ions 48Ca, a stable isotope of calcium, with a natural abundance of 0.2% and eight neutrons more than the conventional isotope.
For Ts, the ions 48Ca reacted with the isotope 249Bk, an actinide. Thus, initially, the 297Ts, which rapidly decayed and lost three or four neutrons, forming the isotopes 294Ts and 293Ts.
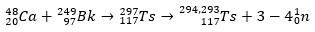
It was possible to verify all this with the analysis of α decay chains, which reached the dubnium and roentgen. As the Ts isotopes obtained are unstable, they spontaneously undergo α-decay reactions, or that is, they emit an α particle (which has two protons and two neutrons) until they reach stable nuclei.
With the decay trail, scientists were able to piece together the puzzle and thus confirm the existence of the superheavy element. for the isotope 293Ts, there were three α decays until the 281Rg, while for the isotope 294Ts were six α decays to the 270DB
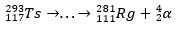
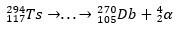
history of teneso
Element 117, the first time, was made through a large international cooperation between Russian and American scientists, which took place at the premises of the Flerov Laboratory for Nuclear Reactions (FLNR), located at the Joint Institute for Nuclear Research, in the city of Dubna, Russia.
It is noteworthy that independently, the results were further confirmed by German scientists from the Helmhotz Center for Research on Heavy Ions (GSI), located in Darmstadt, Germany. During 70 days, in 2009, the team of scientists at the FLNR reacted ions of 48Ca with atoms of 249Bk to thus obtain six atoms of element 117. Then, in 2012, scientists managed to obtain seven atoms of element 117.
The independent confirmation by the GSI was due to another attempt: scientists were trying to produce element 119, which would open the eighth period of the Periodic Table. In this case, the idea was to react an ion of 50Thee with a target of 249bk. However, despite efforts, this element was not detected after four months of attempts.
Changing the titanium ions by 48Ca, GSI scientists went in search of a rare but known superheavy element in order to verify their experimental procedures. Thus, they ended up synthesizing element 117, which served for this element to be confirmed by Iupac.
THE The name tenesso is a reference to the US state of Tennessee.This was a way not only to honor the origin of some scientists involved in the FLNR experiments, but also to remember the place where the isotopes of 249Bk, so crucial to the discovery, were synthesized as they were produced at the Oak Ridge National Laboratory. In English, the element name is tennessine, whose suffix accompanies the other halogens: fluorine, chlorine, bromine, iodine, and astatine.
Solved exercises on teneso
question 1
Teneso, symbol Ts, is the element most recently included in the group of halogens (group 17). Therefore, it is expected that, based on the periodic properties, it has a chemical behavior similar to that of the elements of this group. Thus, among the following alternatives, it is possible to state that teneso:
A) has six valence electrons.
B) has the smallest atomic radius among the elements in this group.
C) has the lowest electronegativity among the elements in this group.
D) needs three electrons to reach the full octet.
E) has the highest electron affinity of group 17.
Resolution:
Alternative C
Ts has, like all elements of group 17, seven electrons in the valence layer, having as valence layer the 7s layer2 7p5. Thus, it can be concluded that it would need an electron to reach the octet, as it has seven electrons in its valence shell.
As the element with the highest number of electron shells among the halogens, Ts also has the highest atomic radius, which guarantees less electron affinity, since the added electrons would be quite far from the nucleus. The smallest radius also causes tennesso to have the lowest electronegativity of all group 17 elements.
question 2
Teneso, symbol Ts and atomic number 117, was first detected by the formation of two of its isotopes: mass 293 and mass 294. Thus, it is possible to say that the number of neutrons in the 293Ts and from 294Ts is equal to, respectively:
A) 293 and 294
B) 117 and 118
C) 177 and 294
D) 176 and 177
E) 176 and 293
Resolution:
Alternative D
The number of neutrons of the two isotopes can be determined as:
A = Z + n
A is the number of pasta atomic, Z the number of protons (atomic number) and n is the number of neutrons.
Substituting for the 293 isotope, we have:
293 = 117 + n
n = 293 - 117
n = 176
For isotope 294, we have:
294 = 117 + n
n = 294 - 117
n = 177
By Stefano Araújo Novais
Chemistry teacher