THE hassium, with symbol Hs and atomic number 108, is one of the elements known as transactinides. Like all elements of this group, it is not found in nature, being synthetic, in addition to being radioactive and unstable. His synthesis is credited to the German laboratories of the Helmholtz Center for Research on Heavy Ions (GSI), in the city of of Darmstadium, Germany, and its name was given in honor of the German state of Hesse, located in the central region of the parents.
Hassium is the heaviest element to have its properties analyzed, through experiments carried out by the GSI itself. The results were important to confirm the position of Hs in the Periodic table, as the similarity between this element and osmium and ruthenium, lighter elements of group 8, was perceived.
Read too:Bohrium — the synthetic chemical element named after Niels Bohr
Hassium Summary
It is a synthetic chemical element located in group 8 of the Periodic Table.
It was synthesized by the Gesellschaft für Schwerionenforschung (GSI) in Darmstadium, Germany.
It is a radioactive element and unstable.
Theoretical and experimental data confirm that its properties resemble the lightest elements of its group.
As a transactinide, it cannot be produced on a large scale, other than being produced at the rate of a few atoms.
Hassium properties
Symbol: hs
Atomic number: 108
Atomic mass: 277 c.u.
Electronic configuration: [Rn] 7s2 5f14 6d6
Most stable isotope: 269Hs (14 second half-life)
Chemical series: group 8; transactinides; super heavy elements.
Hassium features
Hassium is a synthetic element and the heaviest of group 8. It is considered a transactinide, precisely because it appears in the Periodic Table after the actinide series. Like all transactinides, hassium is an element radioactive and unstable.
This means that its isotopes reach little time of half life (the time required for the mass of the atomic species to fall by half). Currently, seven isotopes of hassium are known, whose mass varies from 264 a.m.u. to 270 a.m.u. Among all, the most stable is the isotope 269Hs, with a half-life of 14 seconds.
Although the short half-life makes it difficult to study the chemical properties of transactinides, hassium has had a bit more luck in this regard. In June 2001, it was announced that he had become the heaviest element to have its properties analyzed.
A team managed, in the city of Darmstádio, Germany, to produce about six Hs atoms with a half-life of approximately 10 seconds. Although it seems little, this was enough to confirm that the hassium oxide, HsO4, has strong similarities with the oxides of the lighter elements of group 8, RuO4 and OsO4, that is, the ruthenium it's the osmium.
See too: Seaborgium — another synthetic chemical element with radioactive properties and a short half-life
Obtaining Hassium
Transactinides have in common the difficulty of being produced. In general, state-of-the-art equipment is required, such as particle accelerators. In these, ionic species collide with elements of high atomic mass to form the superheavy elements (in which Hs is included).
In the case of hassium, the studies that confirmed its position in group 8 of the Periodic Table involved its production through the bombardment of magnesium-26, with curium-248 as a target.
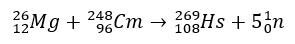
Experiments involving these elements are expensive, and therefore, it is common for theoretical studies to be carried out, precisely to calculate and predict chemical properties. The short half-life is another complicating factor.
The experimental techniques of separation and detection must be very fast for credible results to be produced. Finally, the reaction efficiency of these elements is very low, being in the range of a few atom units.
Precautions with Hassium
Hs is not produced on a large scale, and therefore its manipulation does not involve risks. In addition, it is obtained in research centers with highly controlled environments.
history of hassium

The transactinids were protagonists of a scientific dispute due to the Cold War, a War of Transfers, a name coined in reference to the disputes that involved the production and recognition of elements after fermium (Fm, Z = 100). In this race, the laboratories were involved:
Joint Institute for Nuclear Research, in the city of Dubna, Russia;
Lawrence Berkeley National Laboratory in Berkeley, California;
Gesellschaft für Schwerionenforschung (GSI, better translated as Helmholtz Center for Research on Heavy Ions) in Darmstadium, Germany.
In the case of element 108, the GSI and Dubna groups were involved. However, although the Dubna scientists (led by Yuri Oganessian) first reported the presence of element 108, those from the German group took the credit, as the GSI presented more convincing results, whereas in one experiment he managed to produce three atoms of the isotope 265, while in another he produced one atom of the isotope 264.
These isotopes were produced using the Universal Linear Accelerator (Unilac, better translated as Universal Linear Accelerator), with bombardment of the 208Pb by 58Faith. In 1997, the International Union of Pure and Applied Chemistry (IUPAC) recognized element 108, with symbol Hs, as Hassium in honor of the German state of Hesse.
Exercises solved on hassium
question 1
Hassium, symbol Hs, is considered the heaviest element to have its properties studied experimentally. At the time, researchers were able to determine properties of hasium tetroxide, HsO4. In this species, Hs has the same NOx as the lighter elements of its group, osmium (Os) and ruthenium (Ru). The oxidation number of Hs in hasium tetroxide is equal to:
a) 0
B) +2
C) +4
D) +6
E) +8
Resolution:
Alternative E
THE oxygen, when in a oxide, acquires a charge equal to -2. So, the NOx of Hs, which we will call x, can be calculated as:
x + 4(-2) = 0
x - 8 = 0
x = +8
question 2
The most stable isotope of hassium, Hs, has a half-life of just 14 seconds. That means:
A) in 14 seconds, the mass of the isotope of Hs will have doubled.
B) after 14 seconds, the mass of the Hs isotope will be less than half the initial mass.
C) in just under a minute, the mass of the Hs isotope will be 1/16 of its initial mass.
D) in one minute, the mass of the Hs isotope will be exactly half of its initial mass.
E) in 14 seconds, the mass of the Hs isotope will be divided by 4.
Resolution:
Alternative C
The half-life indicates the time required for the amount of the atomic species to halve. This means that every 14 seconds the amount of Hs drops by half. By observing this half-life, it is clear that, with 56 seconds, four half-lives have already been reached, causing the mass of Hs to be divided by 24, which is 16.
By Stefano Araújo Novais
Chemistry teacher

