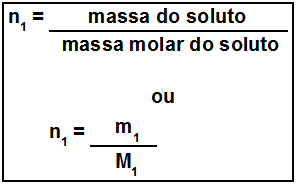
With this class the student will be able to
1. To trace a chronological line on the historical-scientific development of the study of the constitution of matter, from the concept of atom created by Greek philosophers to the current atomic model.
2. Differentiate the atomic models developed, the related theories and the evolution of the representation of the structure of the atom.
3. Be able to identify and differentiate the particles that make up the atom.
1. Discussion about the composition of the matter
Presenting different materials to students and instigating a debate about what they are made of, delving into the characteristics until reaching the common component of all of them: the atom.
2. Theoretical presentation of the topic
Present the evolution of theories for the composition of materials, the concept of atom and the contribution of scientists to the creation of models that represent atoms.
Highlight the differences between atomic models and highlight that information about the atom was discovered and led to the creation of new models.
3. Assessment
Allow the student to put into practice what they have learned using a list of exercises.
1. Evolution of the classification of chemical elements
Present the models used to classify the chemical elements, who were their creators, what elements were known until then and what information they used before reaching the standard current.
2. Presentation of chemical elements
Show students chemical elements, as they are represented in the table and where they can be found in everyday life. Highlight the discovery of some elements, such as phosphorus, the first element discovered, hydrogen, most abundant element in the universe, and mercury, the only metal in a liquid state at room temperature.
3. Atomic number and atom structure
Review the structure of the atom and identify where the protons are located. Explain why the periodic table is arranged in order of increasing atomic number, that is, number of protons.
4. Presentation of the Periodic Table
Highlight that the 118 known chemical elements are distributed in 18 groups and 7 periods of the Periodic Table and the importance of this tool. Define what groups and periods are, presenting the main characteristics.
With this class the student will be able to
1. Identify the concept of chemical bonds and the main types (ionic, covalent and metallic).
2. Explain why atoms unite and how chemical substances are formed.
3. Define the octet rule, explain what the valence of atoms is and its importance for the study of chemical bonds.
4. Recognize, describe and characterize the models of chemical bonds, how they occur and what types of compounds they form.
1. How are chemicals formed?
Use practical, everyday examples to stimulate the development of ideas in the classroom about the composition of materials. You can use table salt and sugar as an example to show the differences between the properties and structures of compounds for students to reflect on until reaching the concept of bonding chemistry.
2. octet theory
Introduce students to the Lewis structure and electronic distribution to represent atoms and facilitate the visualization of the valence shell and valence electrons. Introduce the concept of octet theory and use the noble gas group as an example to compare the its stability and characteristics with elements from other groups, such as alkali metals and halogens.
3. Types of chemical bonds
Define each type of chemical bond and highlight how they occur, what they are for and the types of chemical bonds. Continue using practical examples so that students are able to relate the concepts learned to everyday issues.
The purpose of the class is to present an overview of chemical bonds. Subsequently, specific classes for each type should be taught to delve deeper into the topic.
4. Periodic Table and the study of chemical bonds
Introduce the periodic properties electronegativity and electropositivity and how they are important for making chemical bonds. Show in the Periodic Table where are the elements with the greatest tendency to donate and receive electrons.
1. What is a chemical reaction?
The teacher can use an everyday chemical phenomenon to illustrate a chemical reaction, such as a nail rusting, wood burning, or a pill fizzing, and stimulating student participation in the enumeration of effects that visibly indicate the occurrence of a reaction, such as color change, temperature change, solid formation and release of gases. After that, gather the information and together reach the definition of a chemical transformation.
2. Difference between chemical transformation and physical transformation
Present various phenomena and ask students to classify them as physical and chemical transformation. Focus on observing the composition of materials before and after transformation and emphasize the type of change observed in the structure to distinguish the physical and chemical phenomena regarding the formation or not of new substances.
3. Representation of chemical reactions
Use the Periodic Table to guide how to describe the chemical compounds and, consequently, the chemical elements involved in the reaction.
Start by making generic representations to fix the definition and present the members of a chemical equation. For example, for an addition reaction A and B are reactants and AB is the product:
A + B → AB
Then introduce chemical reactions that actually take place. For this type of reaction, we have the formation of iron II sulfide.
Fe + S → FeS
It should also be stressed the importance of balancing chemical equations and that the number of atoms present in the reactants must be equal to the number of atoms in the product. Also, describe the physical state of the components: solid (s), liquid (l) and gas (g).
4. Laws of chemical reactions
The ponderal laws are the laws that govern chemical reactions and provide guidance on the quantitative aspects of transformations. Therefore, the student must be able to fix the statements of these laws and apply them in the writing of chemical reactions.
5. Types of chemical reactions
Present the different types of chemical reactions through videos, for example, with transformations in everyday life to facilitate the perception of the structure of chemical compounds.
Also, present the conditions for the reactions to occur and where to represent them in the chemical equation, such as light, heat, catalyst, etc.
1. Present different substances and the chemical formula that represents the composition of each one of them. Demonstrate through representation of atoms of chemical elements that substances can be formed by one type of element (simple substances) or by more than one chemical element (substances composites).
2. Recall the concepts of chemical bonds and explain how atoms join together to form ionic compounds (ionic bonding) and molecules (covalent bonds).
3. Remember that in addition to pure substances, the materials we know can also be formed by mixtures (homogeneous or heterogeneous), and present examples so that students are able to differentiate.