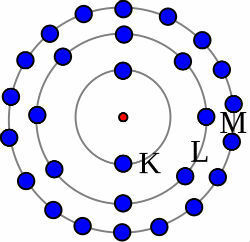
O iron is a transition metal located in group 8 of the Periodic Table, with symbol Fe, atomic number 26 and atomic mass 55.847 u.
Its main characteristics are: shiny metal, with a silvery white appearance, with magnetic properties and that easily rusts in contact with humid environments.
It is the most used metal in the world and the most exported from Brazil. The main use of iron is for the production of steel, used, for example, in civil construction, car manufacturing and household appliances.
Iron is found in ores mainly in the form of oxides and hydroxides. Therefore, commercially used iron is made from the extraction and purification of these materials. The countries with the largest deposits of iron ore are: Australia, Russia, Brazil, China and India.
Iron properties
- Melting point: 1,538 °C
- Boiling point: 2,861 °C
- Physical state at 20 °C: solid
- Density: 7.874 g/cm³
- Oxidation states: +2, +3, +4, +6
- Electronic distribution: [Ar]3d64s2
- Natural isotopes: 56Fe (more stable), 54Faith, 57Faith and 58Faith
Iron uses and applications
The main use of iron is as a raw material for the production of steel, the main metallic alloy of this element and where 98% of the metal extracted from nature is destined.
Steel is a metallic alloy of iron, amounts of carbon ranging from 0.5 to 1.7%, in addition to the addition of small amounts of other chemical elements.
The different types of steel manufactured have different properties because the percentage of carbon is regulated, other elements are included and specific heat treatments are carried out.
Examples of alloys with iron:
- Carbon steel: alloy of iron, carbon, manganese, silicon and traces of other elements;
- Stainless steel: alloy of iron, chromium, carbon and traces of other elements;
- Cast Iron: An alloy of iron, carbon and silicon and may contain traces of other elements.
The first uses of iron were to create tools for agriculture and weapons for hunting and war. Today, iron is present in different everyday materials, such as kitchen utensils, reinforced concrete and beams in construction, means of transport, and much more.
Iron is still used as a catalyst for iron reactions. One of the most produced chemical substances is ammonia, an important raw material for the production of fertilizers.
The combination of hydrogen and nitrogen gases under high temperature and pressure conditions in the synthesis of Haber-Bosch to produce ammonia becomes efficient with the addition of iron as a catalyst to accelerate the reaction.
Occurrence of Iron
Iron (Fe) is the fourth most abundant element in nature, after oxygen, silicon and aluminum. It makes up 5% of the earth's crust and is rarely found free in nature.
The most exploited and most economically important metal ores are:
- Hematite (Fe2O3)
- Magnetite (Fe3O4)
- Taconite (Fe3O3)
- Goethite (Fe2O3.H2O)
- Limonite (2Fe2O3.3H2O)
Although it is estimated that there are more than 300 ores with this element in the composition, those with sufficient iron for processing are in the form of oxides (Ox). Other iron ores are formed by sulfates, silicates and carbonates.
Brazil is the world's second largest producer of iron in the world, after Australia, and the ore of this metal is the most exported from Brazil, with about 68% of the country's exports.
The regions with the greatest exploitation of ore in the Brazilian territory are located in:
- Iron Quadrangle (Minas Gerais)
- Porteirinha (Minas Gerais)
- Mineral Province of Carajás (Pará)
- Corumbá Region (Mato Grosso do Sul)
While in the outermost layer of the Earth iron is the second most abundant metal, in the core of the planet this element has the highest percentage in its composition, since the structure is formed of an iron alloy and nickel.
Therefore, if we consider the planet as a whole, Iron represents approximately 30% of the Earth's chemical composition.
Importance of Iron
Iron is a versatile and important chemical element for chemical, physical and biological processes.
Because it is an abundant metal, easy to obtain, malleable and resistant, iron is widely used for making equipment.
Iron is a metal that is also present in the human body. An adult human has about 2 to 4 g of iron.
It is the main component of hemoglobin in the blood, a protein responsible for transporting oxygen from the lungs to other parts of the body. This is only possible because the iron atom easily binds to oxygen and thus its transport is carried out by the red blood cells.
As iron is important for the production of organic molecules, growth and development of the individual, it is necessary that it be present in the diet.
The recommended intake of iron for adults varies between 10 and 15 mg and can be obtained through mineral-rich foods such as beans, red meats, shellfish, cocoa powder, pumpkin seeds, and spinach.
Iron deficiency in the body causes the most common type of anemia and some of the main symptoms are pallor, fatigue, tachycardia, headache, hair loss and irritability.
Origin and history of Iron
The Age of Metals, is the last phase of prehistory and presents iron as the last metal of the period used by homids, mainly to produce tools and weapons.
The Iron Age took place between 1500 BC. C-300 a. C., but metal objects dating from 3500 BC have already been found. Ç. in Egypt.
The quality of iron was measured by the carbon content present, as compounds of this element were used for iron smelting. Therefore, the lower the amount of carbon, the higher the quality of the iron.
Iron was also the most important metal for the first phase of the Industrial Revolution.
Gain more knowledge with the contents:
- Chemical elements
- metal alloys
- What are metals, examples and properties
Bibliographic references
ATKINS, P.W.; JONES, Loretta. Principles of chemistry: questioning modern life and the environment. 3.ed. Porto Alegre: Bookman, 2006.
FELTRE, Ricardo. Fundamentals of Chemistry: vol. single. 4th ed. Sao Paulo: Moderna, 2005.
Lee, J. D Not so concise inorganic chemistry. Translation of the 5th ed. English. Publisher Edgard Blucher Ltd. 1999.