Electrolysis it is a non-spontaneous process, that is, one that does not occur naturally, in which substances are formed by means of an electrical discharge in compounds melted or dissolved in water. In this process, an electric current reaches a glass container (electrolytic tank) that has two inert electrodes (which do not suffer or oxidation nor reduction) formed by graphite or platinum. These electrodes are connected to an electrical source (usually a drums) and dipped in a salt or base molten or dissolved in water. Just like the battery, electrolysis has a cathode (where reduction occurs) and an anode (where oxidation occurs).
Summary
Electrolysis is a non-spontaneous process;
Simple or composite substances can be formed;
It can occur with a molten solute (igneous electrolysis);
It can occur with a solute dissolved in water (aqueous electrolysis);
A cation always undergoes reduction at the cathode;
An anion always undergoes oxidation at the anode.
See too:Obtaining aluminum from electrolysis

Types of electrolysis
It is an electrolysis that occurs when an electrical discharge is carried out on a molten ionic compound. The ionic compound is one formed by an ionic bond, such as a salt or a base inorganic. When we talk about molten compound, in turn, we refer to the compound that goes from a solid to a liquid state.
- Example of igneous electrolysis
When we perform the fusion of potassium chloride (KCl), this salt undergoes the dissociation process, releasing the potassium cation (K+) and the iodide anion (l-).

Dissociation equation for potassium chloride
When the electric current reaches the electrolytic cell with these ions, the potassium cation is reduced, forming metallic potassium (K), and the iodide anion is oxidized, forming solid iodine (I2).
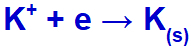
Solid potassium formation equation
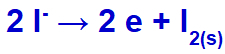
Solid iodine formation equation
It is an electrolysis that occurs when the electrical discharge is carried out on an aqueous solution (has water) formed by a salt or an inorganic base.
- Example of aqueous electrolysis
When we dissolve sodium chloride in water, it dissociates, and the water undergoes ionization:

Water Ionization and Sodium Chloride Dissociation Equations
As sodium cation (Na+) belongs to the IA family, the hydronium cation (H+) passes through the discharge and undergoes reduction, forming hydrogen gas (H2).
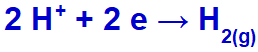
Hydrogen gas formation equation
As chloride (Cl-) is not oxygenated (has no oxygen) and is not fluoride (F-), it is discharged, oxidizing and forming chlorine gas (Cl2).

Chlorine gas formation equation
Selective ion discharge
During an electrolysis process in an aqueous medium (solution formed by water and a solute), we will always have two cations: hydronium, from water, and any other, from the salt dissociation (an example of a solute). We will also have two anions: the hydroxyl, which comes from water, and another one, which comes from salt.

Ions from water ionization and salt dissociation
- Selective discharge for cations and anions
If the cation from the solute belongs to the IA, IIA or IIIA family, the hydronium (H+) will be reduced;
If the cation coming from the solute does not belong to the families mentioned above, it will suffer the reduction.
If the anion from the solute has oxygen in its composition or is a fluoride (F-), the hydroxide (OH-) will undergo oxidation;
If the anion from the solute does not have the above characteristics, it will undergo oxidation.
It is known that water has the ability to self-ionize, producing hydronium ions (H+) and hydroxide (OH-), but this ionization is quite limited. This fact is proven by the inefficiency of pure water in conducting electrical current.
Such as electrolysis involves electrical discharge, to perform the electrolysis of water, it is necessary to dissolve a solute, the which favors the selective discharge of hydronium and hydroxide (as seen in the topic on discharge selective).
If we add sodium sulfate (Na2ONLY4), for example, we will have the sodium cations in the middle (Na+) and hydronium (H+), as well as hydroxide anions (OH-) and sulfate (SO4-2). Thus, when the electric current arrives in the electrolytic tank:
the hydronium will be discharged, reducing, because the sodium belongs to the IA family;
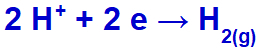
Hydrogen gas formation equation
the hydroxide will undergo discharge, oxidizing, because the sulfate has oxygen in its composition.
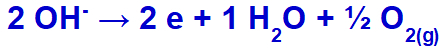
Oxygen gas formation equation
As the two ions (cation and anion) coming from the water were discharged, we say that the electrolysis of the water took place.
See too: Quantitative aspects of electrolysis
Production of simple substances such as hydrogen gas (H2), oxygen gas (O2) etc.;
Production of composite substances such as sodium hydroxide (NaOH), sulfuric acid (H2ONLY4) etc;
Coating a part with a particular metal, such as copper (copper) and gold (gold plating);
Use to remove a certain metal from your ore.
Solved Exercises on Electrolysis
Example 1 - (Vunesp) An aqueous solution of CuCℓ2 is subjected to electrolysis, using platinum electrodes. The correct statement is:
a) Cu ion reduction occurs at the cathode2+.
b) Cu ion oxidation occurs at the anode2+.
c) Chlorine gas is formed at the cathode.
d) Part of the platinum anode dissolves, forming Pt2+.
e) The products of this electrolysis would be different if the electrolysis of CuCl2 was fiery (fusion).
a) Correct. When CuCl2 is dissolved in water, we have the hydronium cations (H+) and copper II (Cu+2). As the copper II cation does not belong to the IA, IIA and IIIA families, it is reduced.
b) False, as the chloride anion (Cl) is oxidized at the anode-), which does not have oxygen in its composition and is not fluoride (F-).
c) False, because the reduction of the copper II cation occurs at the cathode, therefore, there is the formation of the copper metal.
d) False, as platinum or graphite electrodes in electrolysis only conduct electricity, they do not participate in the process.
e) False, as the igneous electrolysis of copper chloride II (CuCl2) would present exactly the same products as the aqueous one, as the ions present would be copper II (Cu+2) and the chloride (Cl-1).
Example 2- (UFRN) Consider the following systems:
I. Molten sodium chloride;
II. Sodium chloride aqueous solution;
III. Fused sodium hydroxide;
IV. Sodium hydroxide aqueous solution.
Those that can provide sodium, when subjected to electrolysis, are:
a) only I and II.
b) only I and III.
c) only II and IV.
d) only III and IV.
e) I, II, III and IV.
The systems that supply metallic sodium as product at the cathode are I and III.
I- The only cation present in the medium is the sodium cation, therefore, only it can reduce, forming metallic sodium.
II- This system does not provide metallic sodium because the sodium chloride was dissolved in water, therefore, in the middle we have the presence of sodium cations (Na+) and hydronium (H+) - this is the one who suffers the reduction, as sodium belongs to the IA family.
III- The only cation present in the medium is the sodium cation (because the material has been subjected to a fusion), therefore, only it undergoes reduction, forming metallic sodium;
IV- This system does not provide metallic sodium because the sodium hydroxide was dissolved in water, so in the middle we have the presence of sodium cations (Na+) and hydronium (H+) - this is the one who suffers the reduction, as sodium belongs to the IA family.
By Me. Diogo Lopes Dias