You amphoteric oxides they are oxides that have an ambiguous behavior, because against an acid they behave like a basic oxide; and in the presence of a base they behave like acidic oxides.
Both the base and the acid with which the amphoteric oxides react must be strong, with a very pronounced chemical character.
Amphoteric oxides are generally ionic solids, poorly soluble in water, and when they react with these strong acids and bases, they produce water and salt.
Generally speaking, we have:
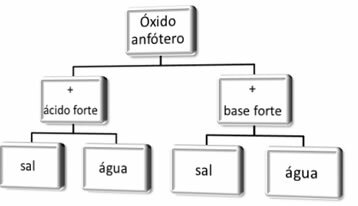
Reaction scheme of amphoteric oxides.
The following are reactions involving two amphoteric oxides, zinc oxide (ZnO) and aluminum oxide (Al2O3). Note how in certain situations they react as acids and in others they present themselves as bases:
1st case: when reacting with strong acids: behave like basic oxides, producing salt and water
Oxide Acid Salt Water
strong amphoteric
(base)
ZnO(s) + H2ONLY4(aq) → ZnSO4(aq) + H2O(1)
Do not stop now... There's more after the advertising ;)
Al2O3(s) + 6 HCl(here) → 2 AlCl3(aq) + 3 H2O(1)
2nd case: when reacting with strong bases: behave like acidic oxides, producing salt and water
Oxide Base Salt Water
strong amphoteric
(acid)
ZnO(s) + 2NaOH(here) → In2ZnO2(aq) + H2O(1)
Al2O3(s) + 2 KOH(here) → 2 KAlO2(aq) + H2O(1)
The main amphoteric oxides are zinc and aluminum; including zinc oxide (ZnO), also known as alvaid, is a white, amorphous, odorless powder, insoluble in water, but soluble in acidic solutions, which is used to paint clown faces. It is also used as an astringent and sunscreen in lotion, ointment and gelatin forms.

Clown applying whitewash (zinc oxide) as makeup
There are also oxides of other metals (SnO and SnO2, PbO and PbO2) and non-metals (As2O3, At2O5, sat2O3, sat2O3).
By Jennifer Fogaça
Graduated in Chemistry
Would you like to reference this text in a school or academic work? Look:
FOGAÇA, Jennifer Rocha Vargas. "Amphoteric oxides"; Brazil School. Available in: https://brasilescola.uol.com.br/quimica/oxidos-anfoteros.htm. Accessed on June 28, 2021.


