Buffer solution it is a homogeneous mixture that does not change pH or pOH when small amounts of strong acid or strong base are added to this mixture. However, the mixture does not change pH or pOH only if it presents one of the two compositions below:
Plug acid (pH less than 7):
It has weak acid mixed with a salt soluble that has the same anion, such as hydrocyanic acid (HCN) and potassium cyanide (KCN), which have the same cyanide anion (CN).
Basic buffer (pH greater than 7):
base weakly mixed with a salt that has the same cation, such as zinc hydroxide [Zn(OH)2] and zinc chloride (ZnCl2), which have the same zinc cation (Zn+2).
It is important, in order to understand the influence that a buffer solution has on receiving a strong acid or base, to remember when acids and bases are considered strong:
→ Ranking of bases in terms of strength
strong: bases with chemical elements from the IA (alkali metals) and IIA (alkaline earth metals) families, with the exception of magnesium;
weak: bases that have the element magnesium and another, as long as it does not belong to the IA and IIA families.
→ Classification of acid for strength
a) For hydrates (oxygen-free acids):
strong: only HCl, HBr and HI;
moderates: HF only;
weak: any other hydroxide.
b) For oxyacids (acids with oxygen):
strong: when the subtraction of the number of oxygens by the number of hydrogens is equal to or greater than 2;
moderates: when the subtraction of the number of oxygens by the number of hydrogens is equal to 1;
weak: when the subtraction of the number of oxygens by the number of hydrogens is equal to or less than 0.
Influence of adding a strong base to a buffer
To explain, let's use the buffer solution formed by zinc hydroxide [Zn(OH)2] and the soluble salt of zinc chloride (ZnCl2), which will receive a small amount of strong sodium hydroxide (NaOH) base. See the balances present in this buffer solution:
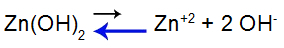
Base dissociation equilibrium equation
Balance shifts to the left because the base is weak.
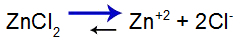
Salt dissociation equilibrium equation
Do not stop now... There's more after the advertising ;)
The balance shifts to the right because salt is soluble.
The strong base has the following balance:
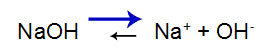
Base dissociation equilibrium equation
Balance shifts to the right because the base is strong.
The added strong base releases the hydroxide anion (OH) into water-), which has a high affinity for zinc cations (Zn+2) from the salt in the second equilibrium. The association between hydroxide and zinc forms zinc hydroxide:
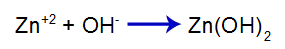
base formation equation
So the OH- of base decreases the amount of zinc in the second equilibrium and increases the amount of base [Zn(OH)2], causing it to dissociate further and release Zn cations+2 in the first equilibrium. As we are not going to have a change in the amount of hydroxide in the medium, therefore, the pH does not change.
Influence of adding a strong acid to a buffer
To explain, let's use the buffer formed by zinc hydroxide [Zn(OH)2] and by the soluble salt of zinc chloride (ZnCl2), which will receive a small amount of hydriodic acid (HI), which is strong. See the balances present in the cap:
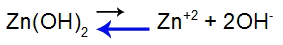
Base dissociation equilibrium equation
Balance shifted to the left because the base is weak.
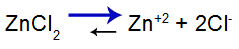
salt balance equation
Balance shifted to the right because salt is soluble.
Strong acid has the following balance:
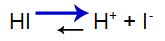
Acid Balance Equation
Balance shifted to the right because the acid is strong.
The added acid produces the hydronium cation in water (H+), which has a high affinity for hydroxide ions (OH-) from the base. The association between hydronium and hydroxide forms a water molecule:
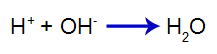
water formation equation
So the H+ of acid decreases the amount of hydroxides in the first equilibrium, increasing base dissociation. As we are not going to have a change in the amount of hydroxide in the medium, therefore, the pH does not change.
By Me. Diogo Lopes Dias
Would you like to reference this text in a school or academic work? Look:
DAYS, Diogo Lopes. "What is a buffer solution?"; Brazil School. Available in: https://brasilescola.uol.com.br/o-que-e/quimica/o-que-e-uma-solucao-tampao.htm. Accessed on July 27, 2021.