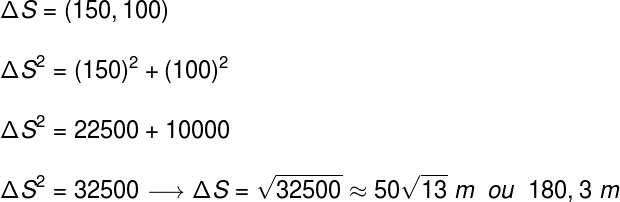THE pastaatomic, as its name suggests, it is the mass of an atom, usually symbolized by “mThe” or “MA”. However, since the atom is an extremely small entity, invisible to human eyes, it is not possible to measure its mass using scales, for example. So scientists determined the mass of atoms by comparing them to the masses of other atoms.
This is done with the other units as well. For example, the unit of mass is the standard kilogram, which corresponds to a cylinder 3.917 cm in height and diameter, made of 10% iridium and 90% platinum. So it serves as a comparison. For example, if we “weigh” an object on a scale and we find that its mass is 10 kg, that means its mass is 10 times greater than the chosen standard: 1 kg.
Do not stop now... There's more after the advertising ;)
The same applies to atoms. The standard chosen was carbon-12, and this atom was arbitrarily assigned a mass of 12 u. The “u” is the unit of atomic mass and is therefore equivalent to 1/12 the mass of a carbon atom.
For example, when we say that the atomic mass of hydrogen is equal to 1 u, it means that it is as if if we divide the carbon into 12 parts, the mass of one of these parts is equivalent to the mass of the atom of hydrogen. Another example is sulfur, its atomic mass is 32 u, which means that its mass is 32 times greater than 1/12 of the mass of
12Ç.1 u = 1/12 of the mass of 1 carbon atom 12
The atomic mass unit is 1/12 the mass of carbon-12
The molecular mass of substances is the sum of the atomic masses of the elements. For example, the atomic mass of oxygen is equal to 16 u, so the molecular mass of the oxygen gas molecule (O2) is equal to 32u.
As laboratory techniques are currently very advanced, there are devices that accurately measure the atomic mass of elements, such as the mass spectrometer. Thus, it is possible to know that 1 u is equal to 1.66054. 10-24 g.
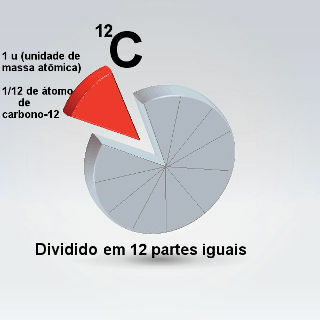
Mind Map: Atomic Mass
*To download the mind map in PDF, Click here!
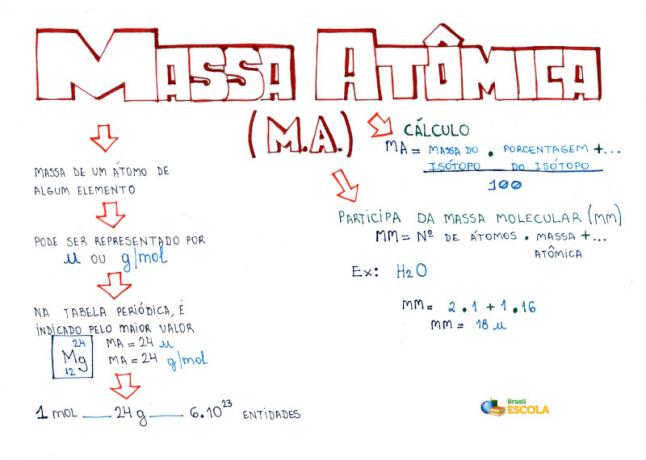
Since atomic masses are determined with great precision, they are given in the periodic table in the form of decimal numbers. But when we go to do the calculations, we usually use the rounded number, with the exception of experiments that need to be very precise. For example, if you look at the Periodic Table, you will see that the atomic mass of oxygen is equal to 15,999 u, but we generally use the value 16 u in the calculations. This happens with carbon too, whose atomic mass is given by 12.01 u, as shown below:
Representation of carbon in the Periodic Table
The elements that appear in the Periodic Table are actually the weighted average of all the element's natural isotopes. Thus, the atomic mass that appears in the Table is also the average of the atomic masses of these elements. For example, chlorine (C?) has two natural isotopes, 35 and 37, whose respective percentages in nature are 75.76% and 24.24%. The atomic masses of each of these isotopes are different, with that of C?-35 being 34.96885 u and that of C?-37 being 36.96590.
In this way, the atomic mass of the element is calculated taking into account the mass of each isotope and the abundance with which it appears in nature. In the case of chlorine, we have:
Atomic mass of the element chlorine = (34,96885. 75,76%) + (36,96590. 24,24%)
100%
Atomic mass of the element chlorine = 35.45
This is the atomic mass value of chlorine that appears on the Periodic Table.
* Mind Map by Me. Diogo Lopes
By Jennifer Fogaça
Graduated in Letters