When a substance is heated, it receives energy in such a way that its molecules are agitated, starting to occupy a greater volume, that is, it undergoes expansion. The opposite occurs when a substance is cooled, as it loses energy and its molecules tend to be very close together, causing a contraction in volume. This causes, normally, matter in the solid state to occupy less volume than when it is in the liquid state.
Contrary to what happens with most substances, water has an anomalous behavior: when it is heated, between the intervals of 0 and 4º C, it suffers contraction and then it starts to dilate, that is, when water is in its solid state, it has a greater volume than in the liquid state in this interval of temperature. This irregular water behavior can be represented with the following graph:
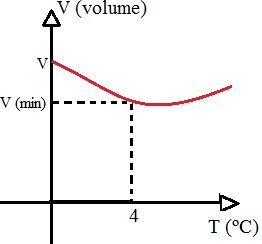
The water volume decreases between 0 and 4°C and then starts to increase
If the volume of water decreases during this interval and its mass is kept constant, consequently the density of water, which is the ratio between mass and volume, will be maximum when the water is at 4ºC. Look at the graphic:
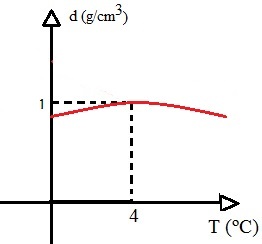
This graph shows that the density of water is maximum when the temperature is 4°C
But what is the reason for the anomalous dilation from water? This explanation is found in their molecular makeup. Water molecules are joined through chemical bonds called Hydrogen bonds.
When the water temperature increases, above 0ºC, the hydrogen bonds start to break, causing an approximation between the molecules. This effect overcomes the molecular thermal agitation, which causes the molecules to move apart and makes them occupy a greater volume in such a way that their volume shrinks.
When the temperature exceeds 4ºC, the number of hydrogen bonds is already very low. In this way, molecular distancing begins to prevail, with, therefore, an increase in its volume.
This irregular water behavior explains several natural phenomena, including the fact that ice floats on water, which is what happens in very cold regions, where the surface of lakes is frozen while at the bottom the water remains liquid. As water has a maximum density at 4ºC, it remains at the bottom, making thermal convection, which is a heat exchange due to the difference in density.
By Mariane Mendes
Graduated in Physics
Source: Brazil School - https://brasilescola.uol.com.br/fisica/a-agua-seu-comportamento-irregula.htm
