The main distinguishing feature of redox reactions is that one chemical species simultaneously loses electrons (oxidizing) and another gains electrons (reducing).
However, in certain reactions it is possible to observe that there is not only an oxidation and/or reduction. Below is an example:
SnS + HCl + HNO3 → SnCl4 + S + NO + H2O
Calculating the oxidation numbers (Nox) of all atoms in this reaction, as taught in the text “Determination of the Oxidation Number (Nox)”, we have:
+2 -2 +1 -1 +1 +5 -2 +4 -1 0 +2 -2 +1 -2
SnS + HCl + HNO3 → SnCl4 + S + NO + H2O
Let's check now which onessubstances suffered variations in their Nox and, consequently, which oxidized and reduced:
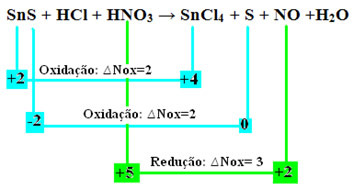
Note that two oxidations have occurred. So, in that case, how to balance the chemical equation?
One important thing to keep in mind that applies to all redox reactions is that the number of electrons given away must always equal the number of electrons received.
Therefore, to balance this type of reaction, follow the same rules established in the text "
Redox balancing”, however, with only one change: the determination of the Nox variation (∆Nox) of the species that generated the two oxidations (SnS) will be the sum of the two ∆Nox, which will give us the total value of lost electrons:- SnS: ∆Total Nox = (∆Nox SnCl4) + (∆Nox S)
SnS: ∆Total NOx = 2 + 2 = 4
- HNO3: ∆Nox = 3
Thus, we can proceed with the following steps for balancing:
- SnS: ∆Total NOx = 4 → 4 will be the HNO coefficient3;
- HNO3: ∆Nox = 3 → 3 will be the coefficient of SnS.
3 SnS + HCl + 4 HNO3 → SnCl4 + S + NO + H2O
Continuing the balancing by trial method:
- Since you have 3 Sn and 3 S in the 1st member, these will also be your coefficients in the 2nd member:
3 SnS + HCl + 4 HNO3 → 3 SnCl4 + 3 Y+NO+H2O
- With that, the 2nd member was left with 12 Cl (3. 4 = 12), so 12 will be the coefficient of the substance in which Cl is found in the 1st member, which is HCl:
3 SnS + 12 HCl + 4 HNO3 → 3 SnCl4 + 3 S + NO +H2O
- We now have 16 H in the 1st member (12 + 4 = 16), hence the coefficient of H2O will be equal to 8, because 8 multiplied by the hydrogen index, which is 2, gives 16:
3 SnS + 12 HCl + 4 HNO3 → 3 SnCl4 + 3 S + NO +8H2O
- Furthermore, in the 1st member there is also 4 N, so 4 will also be the coefficient of N in the 2nd member:
3 SnS + 12 HCl + 4 HNO3 → 3 SnCl4 + 3 S + 4 NO +8 H2O
Note that the amount of oxygen in the 1st limb (3. 4 = 12) is equal to the total amount of that element in the 2nd limb (4 + 8 = 12). So the reaction is balanced.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-com-mais-uma-oxidacao-ou-reducao.htm
