This mixture of gases gives rise to homogeneous mixtures or gaseous solutions. For example, the gases that come out of factory chimneys, or car exhausts, spread through the air atmospheric and, with the passage of time, we were not able to distinguish them any more, as there was a diffusion of these gases through the air.

The gases emitted by the chimneys are diffused and “lost” in the atmospheric air, because the volume of air is much greater than that of the smoke.
| THE diffusion of a gas is a spontaneous movement of a gas through another, that is, its spreading in another gaseous medium. |
| THE effusion of gases is a particular type of diffusion, in which there is leakage of gases through small holes (or porous walls, which is a set of small holes). |
For example, balloons sold to children are filled with helium gas. Over time, this gas ends up passing through the balloon's rubber pores, that is, there is its effusion. This is shown by the fact that the balloon deflates after a few hours.
The Scottish chemist Thomas Graham, in 1829, studied this behavior of gases. He concluded that the speed at which a gas diffuses or effuses in another is related to its density. As his experimental data repeated for all gases, he created the following law that bears his name:
Graham's Law: the rate of diffusion or effusion of a gas is inversely proportional to the square root of its density.
Mathematically, this law can be expressed by the equation:
v α 
Or, relating two different gases, we have:
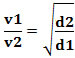
The unit used in this case is the “volume that escapes per unit of time”; therefore generally L/min (liters per minute).
Thus, gases with lower density diffuse faster. If it is in the same temperature and pressure condition, you can also make a relationship of this with the mass gas molar: the greater the density of the gas, the greater its molar mass and the lower its velocity of diffusion; and vice versa. Thus, we have:
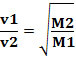
By Jennifer Fogaça
Graduated in Chemistry
Brazil School Team
Source: Brazil School - https://brasilescola.uol.com.br/quimica/difusao-efusao-dos-gases.htm
