The Mechanism is a simulation of the way the reaction proceeds, it describes the steps that the reactants go through to reach the final product.
Mechanisms were proposed based on experiments, as it is not possible to visualize the step by step of a reaction, and are very useful in Organic Chemistry, as organic compounds react with each other in various ways: breaking bonds, forming compounds intermediaries.
There are factors that interfere with the final product of the reaction, such as, for example, the nature of the solvent, polarity of bonds, exchange of electrons, etc. Therefore, a certain mechanism is not always the only way for the formation of the product, they can occur in different ways, ionically or via free radicals, for example.
1. ionic mechanism: process that starts through the heterolytic rupture of a covalent bond and formation of ions (carbocation and carbanion).
Track the Alkene Halogenation Mechanism.
We can divide the mechanism into stages:
1st Stage: Heterolytic disruption of the covalent bond (Cl — Cl): formation of the carbocation and anion (Cl-).
2nd Stage: Chloride anion (Cl-) attack on carbocation.
3rd Stage: Formation of product 1,2 - dichloropropane.
As can be seen, the Halogenation of Alkenes occurs through an ionic mechanism.
Note: As the name implies, Halogenation is done by adding a Halogen (Chlorine) to the molecule.
2. Mechanism via free radicals: the homolytic rupture of a covalent bond forms free radicals (very unstable and reactive), let's look at an example:
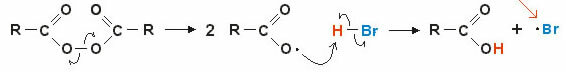
The reaction starts with the breaking of the peroxidic bond and free radical formation. In the intermediate step, the radical attack occurs on the polarized hydrogen of (H — Br) and consequent formation of the new free radical • br (highly unstable and reactive).
By Líria Alves
Graduated in Chemistry
Brazil School Team
See more!
Radicals in Organic Chemistry
Organic chemistry - Chemistry - Brazil School
Source: Brazil School - https://brasilescola.uol.com.br/quimica/mecanismos-reacoes-organicas.htm
