Alcohols can undergo oxidation when exposed to an oxidizing agent, such as an aqueous solution of potassium dichromate (K2Cr2O7) or potassium permanganate (KMnO4) in an acidic medium.
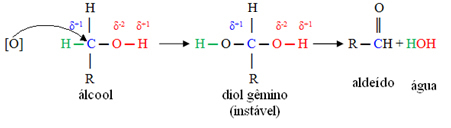
A nascent oxygen [O] in the middle will attack the carbon linked to the alcohol functional group (hydroxyl - OH), forming a very unstable compound, called twin diol, which has two hydroxyls linked to the same carbon. As it is unstable, this compound releases water and gives rise to a new product.
This product will depend on the type of alcohol that has been oxidized, whether it is primary, secondary, tertiary or methanol.
Briefly, we have:
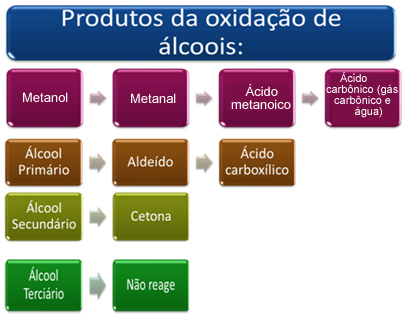
See each case below:
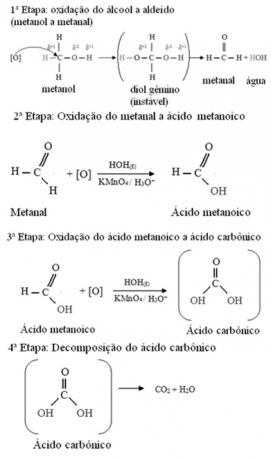
- Methanol (H3C─ OH):
Methanol is the only alcohol that has three hydrogens bonded to carbon that will undergo oxidation. In this case, since there are three points on the molecule that a nascent oxygen can attack, three successive oxidations will occur, as shown in the diagram below:
- Primary alcohols:
In these compounds, the hydroxyl carbon is linked to only one carbon atom, that is, the two other ligands are hydrogens, and there are two places for the nascent oxygen to attack.
First, there will be the formation of an aldehyde, as shown below:
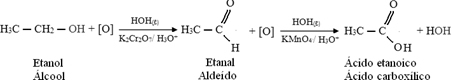
But, oxidation continues, because the reagents used to oxidize alcohol are stronger than those used to oxidize an aldehyde. Then, another nascent oxygen attacks the carbonyl carbon and produces a carboxylic acid.
The following is an example, the oxidation of ethanol, first to ethanal and then to ethanoic acid (acetic acid). This total reaction is the transformation of wine into vinegar.
Partial oxidation of ethanol to ethanal occurs when an alcoholic person takes a disposable breathalyzer test. Inside this device there is a solid mixture of potassium dichromate and silica in an acidic medium, with the following reaction occurring:
K2Cr2O7(aq) + 4H2ONLY4(aq) + 3 CH3CH2oh(g) → Cr2(ONLY4)3(aq) + 7 am2O(1) + 3 CH3CHO(g) + K2ONLY4(aq)
Orangeethanol (colorless)greenethanal (colorless)
Note that, in addition to the oxidation of ethanol (alcohol) to ethanal (aldehyde), there is a simultaneous reduction of dichromate, which is orange, to chromium (III), or even chromium (II), which is green. The change in color will indicate that the person has more alcohol in the blood than allowed.
- Secondary alcohols:
These are compounds in which the hydroxyl carbon is bonded to two other carbon atoms and only one hydrogen atom. Therefore, there will only be one location in the molecule where the nascent oxygen can attack and only one type of product will be formed, which will always be a ketone:
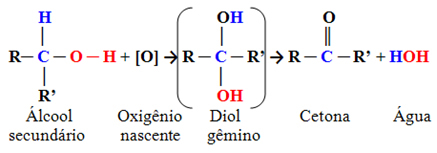
Since the carbonyl carbon of a ketone does not have any hydrogen bonded directly to it, there is no longer any possibility of further oxidation. Therefore, the reaction stops at the ketone.
- Tertiary alcohols:
Tertiary alcohols are those in which the carbon that has the -OH group makes three bonds with other carbon atoms. Since they don't bond with hydrogens, there is no point on the molecule that can be attacked by nascent oxygen. Due to this fact, tertiary alcohols do not undergo oxidation.
* Source and author of the image: CostaPPPR.
By Jennifer Fogaça
Graduated in Chemistry
Source: Brazil School - https://brasilescola.uol.com.br/quimica/oxidacao-dos-alcoois.htm


