At oxidation reactions with secondary alcohols are those in which the interaction between an organic compound from the group of alcohols, which must be secondary, and the so-called Bayer reagent (KMnO4) in an acidic medium.
Whenever Bayer's reagent is in an acidic medium (aq/H+), it undergoes a decomposition reaction, originating potassium oxide, manganese oxide II and nascent oxygens, as we can see in the following equation:
2 kmnO4(aq/H+) → 2 MnO + K2O + 5 [O]
In any oxidation reaction, the nascent oxygens, originated from the decomposition of potassium permanganate in an acidic medium, attack the carbon chains that have pi bonds between carbons (alkenes, alkynes and alkadienes) or that are closed (cyclans, aaromatic cyclenes), generating new chemical compounds.
In the case of oxidation reaction in secondary alcohols, before understanding the reaction mechanism, it is necessary to remember the concept of a secondary alcohol. is called secondary alcohol one in which the hydroxyl (OH) is linked to a
secondary carbon (carbon bonded directly to two other carbon atoms). See the representation of a secondary alcohol: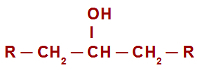
General structural formula of a secondary alcohol
In every oxidation reaction in alcohols, nascent oxygens attack the hydrogens located on the carbon that has the hydroxyl group. In the case of oxidation reactions in secondary alcohols, oxygens have only one hydrogen atom to attack in each structure of this type of alcohol.
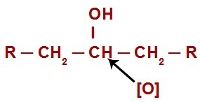
Nascent oxygen attacking a secondary alcohol
When attacking the hydrogen (H) present in the hydroxyl carbon (OH), the nascent oxygen ([O]) forms a new hydroxyl on the same carbon. So we have a gemino diol in jail. As gemino diol (HO – C – OH) is unstable, it decomposes into a water molecule.However, between the carbon and oxygen that is left over from one of the hydroxyls, there is the formation of a double bond (pi and sigma).
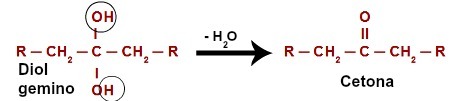
Chemical equation of the oxidation of a secondary alcohol
The product originated from an oxidation reaction of secondary alcohols is always a ketone, for the double bond is always generated at the secondary carbon, which results in a carbonyl between two carbons, thus forming a ketone.
See now a example of the oxidation reaction in secondary alcohols.
Example: Butan-2-ol
O butan-2-ol is a secondary alcohol, since the hydroxyl is attached to a secondary carbon atom. So, on the carbon that has the hydroxyl, there is only one hydrogen. We can check this fact in the structure below:

When subjected to a medium containing water, acid and KMnO4 (Bayer's reagent), the your hydrogen is attacked by a nascent oxygen, transforming into a new hydroxyl, which results in a gemino diol.

Finally, there is a decomposition of the two hydroxyls present in the gemino diol, thus resulting in a water molecule and a ketone, due to the need to create a bond between carbon and one of the oxygens of the decomposed hydroxyls.

we can represent the entire butan-2-ol oxidation reaction by the following equation:

By Me. Diogo Lopes Dias
Source: Brazil School - https://brasilescola.uol.com.br/quimica/reacoes-oxidacao-alcoois-secundarios.htm

